MicroRNA signatures in the pathogenesis and therapy of inflammatory bowel disease
- PMID: 39259390
- PMCID: PMC11390904
- DOI: 10.1007/s10238-024-01476-z
MicroRNA signatures in the pathogenesis and therapy of inflammatory bowel disease
Abstract
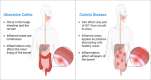
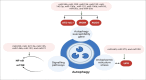
Inflammatory bowel disease (IBD) is a persistent inflammatory illness of the gastrointestinal tract (GIT) triggered by an inappropriate immune response to environmental stimuli in genetically predisposed persons. Unfortunately, IBD patients' quality of life is negatively impacted by the symptoms associated with the disease. The exact etiology of IBD pathogenesis is not fully understood, but the emerging research indicated that the microRNA (miRNA) plays an important role. miRNAs have been documented to possess a significant role in regulating pro- and anti-inflammatory pathways, in addition to their roles in several physiological processes, including cell growth, proliferation, and apoptosis. Variations in the miRNA profiles might be a helpful prognostic indicator and a valuable tool in the differential diagnosis of IBD. Most interestingly, these miRNAs have a promising therapeutic target in several pre-clinical animal studies and phase 2 clinical studies to alleviate inflammation and improve patient's quality of life. This comprehensive review discusses the current knowledge about the significant physiological role of different miRNAs in the health of the intestinal immune system and addresses the role of the most relevant differentially expressed miRNAs in IBD, identify their potential targets, and emphasize their diagnostic and therapeutic potential for future research.
Keywords: Inflammatory bowel disease (IBD); MicroRNA (miRNA); Pathogenesis; Therapy; miRNA signature.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no competing interests to be disclosed.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

