Cancer Trial Eligibility and Therapy Modifications for Individuals With Duffy Null-Associated Neutrophil Count
- PMID: 39259539
- PMCID: PMC11391325
- DOI: 10.1001/jamanetworkopen.2024.32475
Cancer Trial Eligibility and Therapy Modifications for Individuals With Duffy Null-Associated Neutrophil Count
Erratum in
-
Error in Abstract, Results, and Supplement 1.JAMA Netw Open. 2024 Dec 2;7(12):e2456980. doi: 10.1001/jamanetworkopen.2024.56980. JAMA Netw Open. 2024. PMID: 39699902 Free PMC article. No abstract available.
Abstract
Importance: Absolute neutrophil counts (ANCs) are used to determine cancer clinical trial (CCT) eligibility and systemic anticancer therapy (SACT) dose modifications. Duffy null-associated ANC (DANC) is a nonpathologic phenotype associated with lower ANC and most frequently seen in individuals with African and Middle Eastern ancestry. It is unclear whether CCTs exclude or SACT regimens modify doses for individuals with ANC within the DANC reference range.
Objective: To investigate CCT exclusions and SACT dose modifications for ANC within the DANC reference range.
Design, setting, and participants: This cross-sectional study of contemporary CCTs and SACT regimens included adult, interventional, phase 3 CCTs for the 5 most prevalent cancers in the US and United Kingdom (prostate, breast, melanoma, colorectal, and lung cancers) testing SACTs with start dates between November 1, 2021, and November 1, 2023, and that were registered on ClinicalTrials.gov. Preferred curative-intent SACT regimens were listed in National Comprehensive Cancer Network guidelines.
Exposure: Cancer clinical trial exclusions and SACT regimen modifications.
Main outcome and measures: Proportions of CCTs that exclude and SACT regimens that modify doses for individuals with an ANC within the DANC reference range.
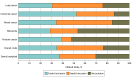
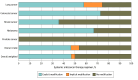
Results: For CCTs, 289 of 382 trials (75.7%) were eligible, of which 221 (76.5% [95% CI, 71.1%-81.2%]) excluded patients with ANC values within the DANC reference range. Colorectal CCTs had the highest (38 of 44 [86.4% (95% CI, 72.6%-94.8%)]) and prostate CCTs had the lowest (11 of 23 [47.8% (95% CI, 26.8%-69.4%)]) proportions of exclusions. Of CCTs testing cytotoxic chemotherapy, 116 of 142 (81.7% [95% CI, 74.3%-87.7%]) had exclusions; 93 of 123 (75.6% [95% CI, 67.0%-82.9%]) CCTs testing targeted therapies alone and 12 of 24 (50.0% [95% CI, 29.1%-70.9%]) testing hormonal therapies alone had exclusions. Among the 113 US- and UK-based trials, exclusions were present in 89 (78.8% [95% CI, 70.1%-85.9%]). Of 71 SACT regimens, 38 (53.5% [95% CI, 41.3%-65.5%]) included dose modifications for ANC values within the DANC reference range. Lung cancer regimens had the highest (23 of 31 [74.2% (95% CI, 55.4%-88.1%)]) and prostate cancer had the lowest (0 of 12 [0 (95% CI, 0%-26.4%)]) proportions of modifications. Regimens including chemotherapy had modifications in 32 of 44 (72.7% [95% CI, 57.2%-85.0%]); 11 of 20 (55.0% [95% CI, 31.5%-76.9%]) of targeted therapy regimens and 0 of 16 (0% [95% CI, 0%-20.6%]) of hormonal therapy regimens had modifications. Among regimens including chemotherapy and/or targeted therapy, modifications were present in 38 of 55 (69.1% [95% CI, 49.7%-73.2%]).
Conclusions and relevance: In this cross-sectional study, substantial proportions of CCTs excluded and SACT regimens modified doses for patients with ANCs in the DANC reference range. These practices structurally discriminate against patients of African and Middle Eastern ancestry. While determining optimal SACT dose modifications requires further study, CCT exclusion criteria should be revised.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

