Iloprost and Organ Dysfunction in Adults With Septic Shock and Endotheliopathy: A Randomized Clinical Trial
- PMID: 39259541
- PMCID: PMC11391323
- DOI: 10.1001/jamanetworkopen.2024.32444
Iloprost and Organ Dysfunction in Adults With Septic Shock and Endotheliopathy: A Randomized Clinical Trial
Abstract
Importance: Soluble thrombomodulin is a marker of endotheliopathy, and iloprost may improve endothelial function. In patients with septic shock, high plasma levels of soluble thrombomodulin (>10 ng/mL) have been associated with worse organ dysfunction and mortality.
Objective: To assess the effects of treatment with iloprost vs placebo on the severity of organ failure in patients with septic shock and plasma levels of soluble thrombomodulin higher than 10 ng/mL.
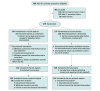
Design, setting, and participants: This investigator-initiated, adaptive, parallel group, stratified, double-blind randomized clinical trial was conducted between November 1, 2019, and July 5, 2022, at 6 hospitals in Denmark. The trial had a maximum sample size of 380, with an interim analysis for futility only at 200 patients with 90 days of follow-up. In total, 279 adults in the intensive care unit (ICU) with septic shock and endotheliopathy were included.
Interventions: Patients were randomized 1:1 to masked intravenous infusion of iloprost, 1 ng/kg/min (n = 142), or placebo (n = 137) for 72 hours.
Main outcomes and measures: The primary outcome was mean daily Sequential Organ Failure Assessment (SOFA) score in the ICU adjusted for trial site and baseline SOFA score for the per-protocol population. SOFA scores for each of the 5 organ systems ranged from 0 to 4, with higher scores indicating more severe dysfunction (maximum score, 20). The secondary outcomes included serious adverse reactions and serious adverse events at 7 days and mortality at 90 days.
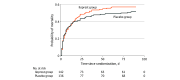
Results: Of 279 randomized patients, data from 278 were analyzed (median [IQR] age, 69 [58-77] years; 171 (62%) male), 142 in the iloprost group and 136 in the placebo group. The trial was stopped for futility at the planned interim analysis. The mean [IQR] daily SOFA score was 10.6 (6.4-14.8) in the iloprost group and 10.5 (5.9-15.5) in the placebo group (adjusted mean difference, 0.2 [95% CI, -0.8 to 1.2]; P = .70). Mortality at 90 days in the iloprost group was 57% (81 of 142) vs 51% (70 of 136) in the placebo group (adjusted relative risk, 1.12 [95% CI, 0.91-1.40]; P = .33). Serious adverse events occurred in 26 of 142 patients (18%) for the iloprost group vs 20 of 136 patients (15%) for the placebo group (adjusted relative risk, 1.25 [95% CI, 0.73-2.15]; P = .52). Only 1 serious adverse reaction was observed.
Conclusions and relevance: In this randomized clinical trial of adults in the ICU with septic shock and severe endotheliopathy, infusion of iloprost, 1 ng/kg/min, for 72 hours did not reduce mean daily SOFA scores compared with placebo. In a clinical context, administration of iloprost will be unlikely to improve outcome in these patients.
Trial registration: ClinicalTrials.gov Identifier: NCT04123444.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

