Discovery and Characterization of Brigimadlin, a Novel and Highly Potent MDM2-p53 Antagonist Suitable for Intermittent Dose Schedules
- PMID: 39259562
- PMCID: PMC11612618
- DOI: 10.1158/1535-7163.MCT-23-0783
Discovery and Characterization of Brigimadlin, a Novel and Highly Potent MDM2-p53 Antagonist Suitable for Intermittent Dose Schedules
Abstract
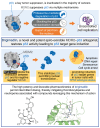
p53 is known as the guardian of the genome and is one of the most important tumor suppressors. It is inactivated in most tumors, either via tumor protein p53 (TP53) gene mutation or copy number amplification of key negative regulators, e.g., mouse double minute 2 (MDM2). Compounds that bind to the MDM2 protein and disrupt its interaction with p53 restore p53 tumor suppressor activity, thereby promoting cell cycle arrest and apoptosis. Previous clinical experience with MDM2-p53 protein-protein interaction antagonists (MDM2-p53 antagonists) has demonstrated that thrombocytopenia and neutropenia represent on-target dose-limiting toxicities that might restrict their therapeutic utility. Dosing less frequently, while maintaining efficacious exposure, represents an approach to mitigate toxicity and improve the therapeutic window of MDM2-p53 antagonists. However, to achieve this, a molecule possessing excellent potency and ideal pharmacokinetic properties is required. Here, we present the discovery and characterization of brigimadlin (BI 907828), a novel, investigational spiro-oxindole MDM2-p53 antagonist. Brigimadlin exhibited high bioavailability and exposure, as well as dose-linear pharmacokinetics in preclinical models. Brigimadlin treatment restored p53 activity and led to apoptosis induction in preclinical models of TP53 wild-type, MDM2-amplified cancer. Oral administration of brigimadlin in an intermittent dosing schedule induced potent tumor growth inhibition in several TP53 wild-type, MDM2-amplified xenograft models. Exploratory clinical pharmacokinetic studies (NCT03449381) showed high systemic exposure and a long plasma elimination half-life in patients with cancer who received oral brigimadlin. These findings support the continued clinical evaluation of brigimadlin in patients with MDM2-amplified cancers, such as dedifferentiated liposarcoma.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Gollner reports grants from the Austrian Research Promotion Agency during the conduct of the study, as well as patent for WO2016001376A1, WO2017060431A1, WO2016026937A1, and WO2015155332A1 issued. D. Rudolph reports grants from Boehringer Ingelheim during the conduct of the study and full-time employment with Boehringer Ingelheim. U. Weyer-Czernilofsky reports grants from Austrian Research Promotion Agency FFG during the conduct of the study. R. Baumgartinger reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study and full-time employment with Boehringer Ingelheim. P. Jung reports grants from the Austrian Research Promotion Agency FFG and personal fees from Boehringer Ingelheim RCV during the conduct of the study, as well as personal fees from Boehringer Ingelheim RCV outside the submitted work. H. Weinstabl reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as a patent for WO2017060431 issued. J. Ramharter reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as a patent for WO 2017/060431 issued. R. Grempler reports grants from the Austrian Research Promotion Agency FFG outside the submitted work, a patent for Boehringer Ingelheim pending and issued, employment with Boehringer Ingelheim, as well as additional fund from the Austrian Research Promotion Agency FFG more than 3 years from 04/2011 to 03/2014 (grants 832260, 837815, 842856). J. Quant reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study. J. Rinnenthal reports grants from the Austrian Research Promotion Agency outside the submitted work, as well as full-time employment with Boehringer Ingelheim RCV GmbH & Co KG from 2012 until 2020. A.P. Pitarch reports a patent for CA3226022A1 pending. B. Golubovic reports grants from the Austrian Research Promotion Agency FFG (grants 832260, 837815, 842856) during the conduct of the study, as well as full-time employment with Boehringer Ingelheim. D. Gerlach reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as full-time employment with Boehringer Ingelheim RCV. G. Bader reports personal fees from Boehringer Ingelheim and grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as personal fees from Boehringer Ingelheim outside the submitted work. K. Wetzel reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, no other relationships/conditions/circumstances that present a potential conflict of interest, as well as employment with Boehringer Ingelheim. S. Otto reports grants from the Austrian Research Promotion Agency during the conduct of the study. C. Mandl reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as full-time employment with Boehringer Ingelheim RCV GmbH & Co KG. G. Boehmelt reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study. D.B. McConnell reports financial support by the Austrian Research Promotion Agency FFG more than 3 years from 04/2011 to 03/2014 (grants 832260, 837815, 842856). N. Kraut reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as full-time employment with Boehringer Ingelheim. P. Sini reports grants from the Austrian Research Promotion Agency FFG during the conduct of the study, as well as personal fees from Boehringer Ingelheim outside the submitted work.
Figures





References
-
- Lane DP. Cancer. p53, guardian of the genome. Nature 1992;358:15–6. - PubMed
-
- Gottlieb TM, Oren M. p53 and apoptosis. Semin Cancer Biol 1998;8:359–68. - PubMed
-
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 1991;352:345–7. - PubMed
-
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. . Restoration of p53 function leads to tumour regression in vivo. Nature 2007;445:661–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

