Intrathecal Drug Delivery System in Prepontine Cistern for Patients with Intractable Craniofacial Cancer Pain: A Multicenter Retrospective Study
- PMID: 39259695
- PMCID: PMC12220574
- DOI: 10.1213/ANE.0000000000007262
Intrathecal Drug Delivery System in Prepontine Cistern for Patients with Intractable Craniofacial Cancer Pain: A Multicenter Retrospective Study
Abstract
Background: Patients with craniofacial cancer frequently suffer from severe pain. The traditional intrathecal, oral, or intravenous analgesics could only provide insufficient pain relief with many side effects. Thus, a more effective analgesia approach is required. This study aimed to investigate the safety and efficacy of placing the catheter of an intrathecal morphine pump in the prepontine cistern for the treatment of craniofacial cancer pain.
Methods: We performed a retrospective study of patients with primary or metastatic craniofacial cancer pain who received the catheter placement of an intrathecal morphine pump into the prepontine cistern in eleven medical centers from September 2019 to December 2023. Friedman test and pairwise signed-rank test were used to evaluate the difference in numeric rating scale (NRS) scores, the number of breakthrough pain episodes, dose of intrathecal morphine, and dose of systemic morphine equivalents (oral, patch, intravenous) from preoperative period to postoperative days 1, 7, and 30. P values were corrected for multiple comparisons using Bonferroni test.
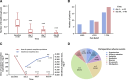
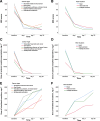
Results: The study included 33 patients. The median (interquartile range [IQR]) of NRS scores at days 1, 7, and 30 postimplant were 2.0 (1.0-3.5), 2.0 (1.0-2.0), and 1.0 (1.0-2.0), respectively, which was significantly lower than that before surgery (median, 8.0; IQR, 7.0-10.0; all P < .001). Compared to baseline number/d of breakthrough pain episodes (median, 6.0; IQR, 4.5-10.0), there was a progressive decrease in the number/d of breakthrough pain episodes at day 1, day 7, and day 30 postimplant, and the median (IQR) were 1.0 (0.0-3.0), 2.0 (0.0-3.0), and 0.0 (0.0-1.2), respectively (all P < .001). Approximately 78.8% and 96.7% of patients reported pain relief >50% at days 1 and 30 postimplant, respectively. Compared with that at day 1 postimplant, the proportion of patients with a pain relief rate >75% at day 30 postimplant also increased with continued intrathecal treatment. Compared to the dose of baseline systemic morphine equivalents (median, 228 mg.d -1 ; IQR, 120-408 mg.d -1 ), the dose of systemic morphine equivalents reduced significantly from 0(0-120) mg.d -1 at day 1 postimplant ( P = .001), to 0 (0-0) mg.d -1 at days 7 and 30 postimplant (both P < .001). Few patients reported perioperative adverse events, including nausea, constipation, hypotension, urinary retention, dry mouth, headache, and sedation. No severe adverse events occurred.
Conclusions: Placing the catheter tip of an intrathecal morphine pump into the prepontine cistern could effectively relieve refractory craniofacial cancer pain with an extremely low total morphine dose requirement and few adverse events. This procedure could be considered in patients with severe refractory craniofacial cancer pain.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Anesthesia Research Society.
Conflict of interest statement
Conflicts of Interest, Funding: Please see DISCLOSURES at the end of this article.
Figures



References
-
- Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. - PubMed
-
- Porter LS, Keefe FJ. Psychosocial issues in cancer pain. Curr Pain Headache Rep. 2011;15:263–270. - PubMed
-
- Gough N, Miah AB, Linch M. Nonsurgical oncological management of cancer pain. Curr Opin Support Palliat Care. 2014;8:102–111. - PubMed
-
- Van den Beuken-van Everdingen M, De Rijke J, Kessels A, Schouten H, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. - PubMed
-
- Stjernswärd J. WHO cancer pain relief programme. Cancer Surv. 1988;7:195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

