T cell responses to repeated SARS-CoV-2 vaccination and breakthrough infections in patients on TNF inhibitor treatment: a prospective cohort study
- PMID: 39260039
- PMCID: PMC11416219
- DOI: 10.1016/j.ebiom.2024.105317
T cell responses to repeated SARS-CoV-2 vaccination and breakthrough infections in patients on TNF inhibitor treatment: a prospective cohort study
Abstract
Background: Understanding cellular responses to SARS-CoV-2 immunisations is important for informing vaccine recommendations in patients with inflammatory bowel disease (IBD) and other vulnerable patients on immunosuppressive therapies. This study investigated the magnitude and quality of T cell responses after multiple SARS-CoV-2 vaccine doses and COVID-19 breakthrough infection.
Methods: This prospective, observational study included patients with IBD and arthritis on tumour necrosis factor inhibitors (TNFi) receiving up to four SARS-CoV-2 vaccine doses. T cell responses to SARS-CoV-2 peptides were measured by flow cytometry before and 2-4 weeks after vaccinations and breakthrough infection to assess the frequency and polyfunctionality of responding cells, along with receptor-binding domain (anti-RBD) antibodies.
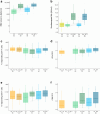
Findings: Between March 2, 2021, and December 20, 2022, 143 patients (118 IBD, 25 arthritis) and 73 healthy controls were included. In patients with either IBD or arthritis, humoral immunity was attenuated compared to healthy controls (median anti-RBD levels 3391 vs. 6280 BAU/ml, p = 0.008) after three SARS-CoV-2 vaccine doses. Patients with IBD had comparable quantities (median CD4 0.11% vs. 0.11%, p = 0.26, CD8 0.031% vs. 0.047%, p = 0.33) and quality (polyfunctionality score: 0.403 vs. 0.371, p = 0.39; 0.105 vs. 0.101, p = 0.87) of spike-specific T cells to healthy controls. Patients with arthritis had lower frequencies but comparable quality of responding T cells to controls. Breakthrough infection increased spike-specific CD8 T cell quality and T cell responses against non-spike peptides.
Interpretation: Patients with IBD on TNFi have T cell responses comparable to healthy controls despite attenuated humoral responses following three vaccine doses. Repeated vaccination and breakthrough infection increased the quality of T cell responses. Our study adds evidence that, in the absence of other risk factors, this group may in future be able to follow the general recommendations for COVID-19 vaccines.
Funding: South-Eastern Norway Regional Health Authority, Coalition for Epidemic Preparedness Innovations (CEPI), Norwegian Institute of Public Health, Akershus University Hospital, Diakonhjemmet Hospital.
Keywords: Arthritis; Inflammatory bowel disease; SARS-CoV-2; T cells; TNF inhibitors; Vaccination.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests KHB reports funding from Akershus University Hospital and speaker bureaus for Janssen-Cilag. TKK reports grants from AbbVie, BMS, Galapagos, Novartis, Pfizer, UCB, speakers' bureaus from Grünenthal, Janssen, Sandoz, consultant fees from AbbVie, Gilead, Janssen, Novartis, Pfizer, Sandoz, UCB, and participation on advisory board for AbbVie. JJ reports grants from Boehringer-Ingelheim, speakers bureaus from AbbVie/Abbott, Bristol-Myers, Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, Takeda, consultant fees from AbbVie/Abbott, Pfizer, and participation in advisory board for AbbVie/Abbott, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, Takeda. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the university of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations (CEPI), speakers' bureaus from Incyte, Janssen, and expert testimony for Norwegian Medicines Agency. EAH reports speakers' bureaus from Pfizer, UCB, Novartis, and consulting fees from Abbvie, Pfizer, Eli Lilly. GG reports speaker bureaus from AbbVie/Abbott, Galapagos, Pfizer, UCB, participation in advisory board from AstraZeneca, Janssen, Moderna, Seqirus. and consulting fees from The Norwegian System of Patient Injury Compensation. JTV reports grant from CEPI. FLJ reports funding from South-East Health Authorities in Norway, CEPI and Oslo University Hospital. GLG reports speakers' bureaus from AbbVie/Abbott, Galapagos, Pfizer, UCB, and participation in advisory board from AbbVie/Abbott, Galapagos, Pfizer, UCB, Novartis. KKJ reports speakers’ bureaus from Bristol-Myers Squibb and Janssen, and participation in advisory board for AstraZeneca and IPSEN. SWS reports participation in advisory board for AstraZeneca. ASW reports travel grant of GBP 250 from the British Society of Immunology to support travelling to the BSI Congress 2023 (4–7th Dec 2023) and presenting a poster with some of this data. HSØ, SB, GS, IFK, IJ, UCN, ABK, IEC, SEJ, KPL, AC, ATT, JS, SAP, HK, and SM report nothing to disclose.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

