Maternal influenza vaccination and associated risk of fetal loss: A claims-based prospective cohort study
- PMID: 39260053
- PMCID: PMC11911014
- DOI: 10.1016/j.vaccine.2024.126256
Maternal influenza vaccination and associated risk of fetal loss: A claims-based prospective cohort study
Abstract
Background: Although numerous studies support the safety of influenza vaccination during pregnancy, fewer studies have evaluated the risk of miscarriage or considered the effect of prior immunization.
Methods: Using national de-identified administrative claims data from the Optum Labs Data Warehouse, we conducted a claims-based cohort study of 117,626 pregnancies between January 2009 and December 2018. We identified pandemic A(H1N1)pdm09 and seasonal influenza vaccinations using CPT codes. Fetal loss was defined as miscarriage, medical termination, or stillbirth as identified by ICD-10-CM diagnostic codes. Cox proportional hazard models treating influenza vaccination as a time-varying exposure, weighted for loss-to-follow-up and stratified by baseline probability of vaccination, were used to model the risk of fetal loss by exposure to influenza vaccine.
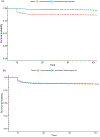
Results: About 31.4 % of the cohort had a record of influenza vaccination; 10.0 % were vaccinated before pregnancy only, 17.8 % during pregnancy only, and 3.6 % before and during pregnancy. The risk of miscarriage was 39 % lower among those vaccinated during pregnancy compared to unvaccinated (adjusted hazard ratio, aHR 0.61; 95 % CI 0.50, 0.74) and was similar for medical termination or stillbirth (HR 0.69; 95 % CI 0.45, 1.03 and aHR 0.99; 95 % CI 0.76, 1.30, respectively). Similar results were observed for women who received the vaccine before and during pregnancy. We observed little to no association between vaccination before pregnancy and risk of miscarriage (HR 0.98; 95 % CI 0.76, 1.26), medical termination (HR 1.02; 95 % CI 0.46, 2.24), or stillbirth (HR 1.14, 95 % CI 0.77, 1.69).
Discussion: Influenza vaccination was not associated with an increased risk of fetal loss. These results support the safety of influenza vaccine administration even when administered before or early during pregnancy.
Keywords: Inactivated influenza vaccine; Maternal vaccination; Miscarriage; Pregnancy; Vaccine safety.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Onyebuchi A Arah reports financial support was provided by Optum Labs Inc. Sheena G Sullivan reports a relationship with Seqirus Australia Pty Ltd. that includes: consulting or advisory. Sheena G Sullivan reports a relationship with Moderna Inc. that includes: consulting or advisory. Sheena G Sullivan reports a relationship with Novavax Inc. that includes: consulting or advisory. Sheena G Sullivan reports a relationship with Pfizer Inc. that includes: consulting or advisory. Annette K Regan reports a relationship with Moderna Inc. that includes: board membership. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012;87:461–76. - PubMed

