Monovalent rotavirus vaccine effectiveness and long-term impact among children <5 years old in Antananarivo, Madagascar, 2010-2022
- PMID: 39260057
- PMCID: PMC11866102
- DOI: 10.1016/j.vaccine.2024.126321
Monovalent rotavirus vaccine effectiveness and long-term impact among children <5 years old in Antananarivo, Madagascar, 2010-2022
Abstract
Background: Monovalent rotavirus vaccine substantially reduced rotavirus disease burden after introduction (May 2014) in Madagascar. We examined the effectiveness and long-term impact on acute watery diarrhea and rotavirus-related hospitalizations among children <5 years old at two hospitals in Antananarivo, Madagascar (2010-2022).
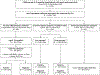
Methods: We used a test-negative case-control design to estimate monovalent rotavirus vaccine effectiveness (VE) against laboratory-confirmed rotavirus hospitalizations among children age 6-23 months with documented vaccination status adjusted for year of symptom onset, rotavirus season, age group, nutritional status, and clinical severity. To evaluate the impact, we expanded to children age 0-59 months with acute watery diarrhea. First, we used admission logbook data to compare the proportion of all hospitalizations attributed to diarrhea in the pre-vaccine (January 2010-December 2013), transition period (January 2014-December 2014), and post-vaccine (January 2015-December 2022) periods. Second, we used active surveillance data (June 2013-May 2022) to describe rotavirus positivity and detected genotypes by vaccine introduction period and surveillance year (1 June-31 May).
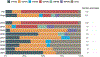
Result: Adjusted VE of at least one dose against hospitalization due to rotavirus diarrhea among children age 6-23 months was 61 % (95 % CI: -39 %-89 %). The annual median proportion of hospitalizations attributed to diarrhea declined from 28 % in the pre-vaccine to 10 % in the post-vaccine period. Rotavirus positivity among hospitalized children age 0-59 months with acute watery diarrhea was substantially higher during the pre-vaccine (59 %) than the post-vaccine (23 %) period. In the pre-vaccine period, G3P[8] (76 %) and G2P[4] (12 %) were the dominant genotypes detected. Although genotypes varied by surveillance year, G1P[8] and G2P[4] represented >50 % of the genotypes detected post-introduction.
Conclusions: Rotavirus vaccine has been successfully implemented in Madagascar's routine childhood immunization program and had a large impact on rotavirus disease burden, supporting continued use of rotavirus vaccines in Madagascar.
Keywords: Genotype; Madagascar; Rotavirus; Rotavirus surveillance; Rotavirus vaccine effectiveness; Rotavirus vaccine impact.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

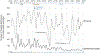


References
-
- WHO. Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009;84:533–40. - PubMed
-
- Rotavirus Vaccine | ViewHub. https://view-hub.org/vaccine/rota. [Accessed 27 February 2024].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

