Biomolecular condensates regulate cellular electrochemical equilibria
- PMID: 39260373
- PMCID: PMC11490381
- DOI: 10.1016/j.cell.2024.08.018
Biomolecular condensates regulate cellular electrochemical equilibria
Abstract
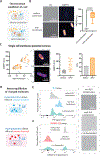
Control of the electrochemical environment in living cells is typically attributed to ion channels. Here, we show that the formation of biomolecular condensates can modulate the electrochemical environment in bacterial cells, which affects cellular processes globally. Condensate formation generates an electric potential gradient, which directly affects the electrochemical properties of a cell, including cytoplasmic pH and membrane potential. Condensate formation also amplifies cell-cell variability of their electrochemical properties due to passive environmental effect. The modulation of the electrochemical equilibria further controls cell-environment interactions, thus directly influencing bacterial survival under antibiotic stress. The condensate-mediated shift in intracellular electrochemical equilibria drives a change of the global gene expression profile. Our work reveals the biochemical functions of condensates, which extend beyond the functions of biomolecules driving and participating in condensate formation, and uncovers a role of condensates in regulating global cellular physiology.
Keywords: antibiotics; biomolecular condensates; electrochemical features of condensates; global cellular physiology; intracellular electrochemistry; ion flux; membrane potential.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



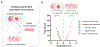
References
-
- Wei M-T, Elbaum-Garfinkle S, Holehouse AS, Chen CC-H, Feric M, Arnold CB, Priestley RD, Pappu RV, and Brangwynne CP (2017). Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nature Chemistry 9, 1118–1125. 10.1038/nchem.2803. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

