Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP type C
- PMID: 39260416
- PMCID: PMC11485244
- DOI: 10.1038/s41586-024-08024-5
Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP type C
Abstract
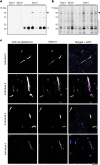
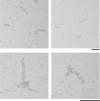
Neurodegenerative diseases are characterized by the abnormal filamentous assembly of specific proteins in the central nervous system1. Human genetic studies have established a causal role for protein assembly in neurodegeneration2. However, the underlying molecular mechanisms remain largely unknown, which is limiting progress in developing clinical tools for these diseases. Recent advances in cryo-electron microscopy have enabled the structures of the protein filaments to be determined from the brains of patients1. All neurodegenerative diseases studied to date have been characterized by the self-assembly of proteins in homomeric amyloid filaments, including that of TAR DNA-binding protein 43 (TDP-43) in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) types A and B3,4. Here we used cryo-electron microscopy to determine filament structures from the brains of individuals with FTLD-TDP type C, one of the most common forms of sporadic FTLD-TDP. Unexpectedly, the structures revealed that a second protein, annexin A11 (ANXA11), co-assembles with TDP-43 in heteromeric amyloid filaments. The ordered filament fold is formed by TDP-43 residues G282/G284-N345 and ANXA11 residues L39-Y74 from their respective low-complexity domains. Regions of TDP-43 and ANXA11 that were previously implicated in protein-protein interactions form an extensive hydrophobic interface at the centre of the filament fold. Immunoblots of the filaments revealed that the majority of ANXA11 exists as an approximately 22 kDa N-terminal fragment lacking the annexin core domain. Immunohistochemistry of brain sections showed the colocalization of ANXA11 and TDP-43 in inclusions, redefining the histopathology of FTLD-TDP type C. This work establishes a central role for ANXA11 in FTLD-TDP type C. The unprecedented formation of heteromeric amyloid filaments in the human brain revises our understanding of amyloid assembly and may be of significance for the pathogenesis of neurodegenerative diseases.
© 2024. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures







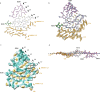



Update of
-
Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP Type C.bioRxiv [Preprint]. 2024 Jun 26:2024.06.25.600403. doi: 10.1101/2024.06.25.600403. bioRxiv. 2024. Update in: Nature. 2024 Oct;634(8034):662-668. doi: 10.1038/s41586-024-08024-5. PMID: 38979278 Free PMC article. Updated. Preprint.
References
-
- Scheres, S. H. W., Ryskeldi-Falcon, B. & Goedert, M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature621, 701–710 (2023). - PubMed
-
- Wilson, D. M. et al. Hallmarks of neurodegenerative diseases. Cell186, 693–714 (2023). - PubMed
-
- Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science314, 130–133 (2006). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG077444/AG/NIA NIH HHS/United States
- RF1 AG071177/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- U01 NS110437/NS/NINDS NIH HHS/United States
- R01 NS085770/NS/NINDS NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- R01 AG056258/AG/NIA NIH HHS/United States
- R01 DC008552/DC/NIDCD NIH HHS/United States
- RF1 NS110437/NS/NINDS NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- R01 AG080001/AG/NIA NIH HHS/United States
- R01 AG071177/AG/NIA NIH HHS/United States
- R01 NS137469/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

