Rotation or change of biotherapy after TNF blocker treatment failure for axial spondyloarthritis: the ROC-SpA study, a randomised controlled study protocol
- PMID: 39260856
- PMCID: PMC11409346
- DOI: 10.1136/bmjopen-2024-087872
Rotation or change of biotherapy after TNF blocker treatment failure for axial spondyloarthritis: the ROC-SpA study, a randomised controlled study protocol
Abstract
Introduction: Axial spondyloarthritis (axSpA) is a chronic inflammatory disease characterised by inflammatory low back pain. Non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as a first treatment in axSpA. In case of inadequate response to NSAIDs, biological disease-modifying antirheumatic drugs (bDMARDs) should be introduced according to the recommendations of the European League Against Rheumatism (EULAR) and the American College of Rheumatology. Until 2015, only bDMARD was recommended for axSpA in case of failure to anti-tumour necrosis factor (TNF). The 2022 Assessment of SpondyloArthritis International Society (ASAS)-EULAR recommendation proposed to start an alternative bDMARD but without advocating a switch in mode of action as proposed in rheumatoid arthritis. Since 2015, the inhibition of interleukin (IL)-17 has demonstrated efficacy in axSpA. Then, we designed a randomised multicentre clinical trial to identify the more effective treatment after a first anti-TNF failure in axSpA, comparing an anti-IL-17 to a second anti-TNF.
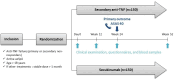
Methods and analysis: The ROC-SpA (Rotation Or Change of biotherapy after first anti-TNF treatment failure in axSpA patients) study is a prospective, randomised, multicentre, superiority open-label phase IV trial comparing an anti-IL-17 strategy (secukinumab or ixekizumab) to a second TNF blocker in a 1:1 ratio. Patients with an active axSpA (Bath Ankylosing Spondylitis Disease Activity Index >4 or ankylosing spondylitis disease activity score (ASDAS) >3.5) with inadequate 3 months response to a first anti-TNF and with a stable dose of conventional synthetic DMARDs, oral corticosteroids and/or NSAIDs for at least 1 month are included in 31 hospital centres in France and Monaco. The primary outcome is the ASAS40 response at week 24. The secondary outcomes are ASAS40 at weeks 12 and 52, other clinical scores (ASAS20, partial remission rate, ASDAS major improvement rate) at weeks 12, 24 and 52 with the drugs and anti-drugs concentrations at baseline, weeks 12, 24 and 52. The primary analysis is performed at the end of the study according to the intent-to-treat principle.
Ethics and dissemination: Ethics approval was obtained from the committee for the protection of persons (Comité de protection des personnes Ouest IV #12/18_1, 6 February 2018) and registered in ClinicalTrials.gov and in EudraCT. Results of this study, whether positive or negative, will be presented at national and international congresses, to national axSpA patient associations and published in a peer-reviewed journal. It could also impact the international recommendation to manage patients with axSpA.
Trial registration number: NCT03445845 and EudraCT2017-004700-22.
Keywords: Clinical Trial; RHEUMATOLOGY; THERAPEUTICS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ED, CM, TB-A, FR, MA, LDA and EP declare no competing interests. AF has received grants or contracts (Eli Lilly, Pfizer, AbbVie, Gilead and BMS), consulting fees (AstraZeneca, AbbVie, Pfizer and Gilead) and honorary payments (BMIS, AbbVie, Eli Lilly, Pfizer and MSD) and participated in advisory boards (Astra-Zeneca, Gilead, Novartis, AbbVie, Eli Lilly, Pfizer, J&J, Mylan and UCB). HM has received grants and contract (Celltrion Healthcare, Lilly, Medac, Nordic Pharma), consulting fees (AbbVie, Accord, Celltrion Healthcare, Johnson and Johnson, UCB) and honorary payments (AbbVie, BMS, Lilly, Pfizer) and participated in advisory boards (Celltrion Healthcare, Eli Lilly, Pfizer, UCB).
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
