Emerging Hybrid Intracoronary Imaging Technologies and Their Applications in Clinical Practice and Research
- PMID: 39260958
- PMCID: PMC11996234
- DOI: 10.1016/j.jcin.2024.07.007
Emerging Hybrid Intracoronary Imaging Technologies and Their Applications in Clinical Practice and Research
Abstract
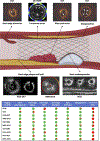
Intravascular ultrasound and optical coherence tomography are used with increasing frequency for the care of coronary patients and in research studies. These imaging tools can identify culprit lesions in acute coronary syndromes, assess coronary stenosis severity, guide percutaneous coronary intervention (PCI), and detect vulnerable plaques and patients. However, they have significant limitations that have stimulated the development of multimodality intracoronary imaging catheters, which provide improvements in assessing vessel wall pathology and guiding PCI. Prototypes combining 2 or even 3 imaging probes with complementary attributes have been developed, and several multimodality systems have already been used in patients, with near-infrared spectroscopy intravascular ultrasound-based studies showing promising results for the identification of high-risk plaques. Moreover, postmortem histology studies have documented that hybrid imaging catheters can enable more accurate characterization of plaque morphology than standalone imaging. This review describes the evolution in the field of hybrid intracoronary imaging; presents the available multimodality catheters; and discusses their potential role in PCI guidance, vulnerable plaque detection, and the assessment of endovascular devices and emerging pharmacotherapies targeting atherosclerosis.
Keywords: hybrid intravascular imaging; intravascular ultrasound; near-infrared fluorescence; near-infrared spectroscopy; optical coherence tomography.
Copyright © 2024 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Prof Jaffer has received research grants from Canon, Siemens, Shockwave, Teleflex, Mercator, Boston Scientific, HeartFlow, and Neovasc; has received consultant/speaker fees from Boston Scientific, Siemens, Magenta Medical, Philips, Biotronik, Mercator, and Abiomed; has equity interest in Intravascular Imaging Inc and DurVena. Massachusetts General Hospital has licensing arrangements with Terumo, Canon, and SpectraWAVE; Dr Jaffer has the right to receive royalties. Prof Serruys has served as a consultant for Merillife, Novartis, Xeltis, SMT, and Philips. Prof Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant for Abbott, Daiichi-Sankyo, Ablative Solutions, CorFlow, Apollo Therapeutics, Cardiomech, Gore, Robocath, Miracor, Vectorious, Abiomed, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Elucid Bio, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, Cardiac Success, and HighLife; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWAVE, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter. Prof Stone’s employer, Mount Sinai Hospital, receives research grants from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo, and V-wave. Prof Muller is a cofounder of, has equity interest in, has served as a consultant for, and is not an employee of SpectraWAVE; and is a cofounder with no financial interest in Infraredx, Inc. Prof Van Soest is a cofounder of, consultant for, and has financial interest in Kaminari Medical BV, which develops IVPA/IVUS technology; and has received research grants from Boston Scientific. Dr Courtney is an employee of; has shareholder/equity interest in Conavi Medical Inc; and has received stock options, royalties, and research funding from Conavi Medical Inc. Prof Tearney has financial/fiduciary interest in SpectraWave, a company developing an OCT NIRS intracoronary imaging system and catheter. His financial/fiduciary interest was reviewed and is managed by Massachusetts General Brigham in accordance with their conflict of interest policies. Prof Tearney has received materials from Terumo Corporation; and has received sponsored research funding from Canon Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Bourantas CV, Tenekecioglu E, Radu M, Raber L, Serruys PW. State of the art: role of intravascular imaging in the evolution of percutaneous coronary intervention - a 30-year review. EuroIntervention. 2017;13:644.–. - PubMed
-
- Holm NR, Andreasen LN, Neghabat O, et al. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med 2023;389(16): 1477.–. - PubMed
-
- Raber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281.–. - PubMed
-
- Stone GW, Christiansen EH, Ali ZA, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet. 2024;403:824.–. - PubMed
-
- Ladwiniec A, Walsh SJ, Holm NR, et al. Intravascular ultrasound to guide left main stem intervention: a NOBLE trial substudy. EuroIntervention. 2020;16:201.–. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

