Spectroscopic definition of ferrous active sites in non-heme iron enzymes
- PMID: 39261000
- PMCID: PMC11391101
- DOI: 10.1016/bs.mie.2024.05.019
Spectroscopic definition of ferrous active sites in non-heme iron enzymes
Abstract
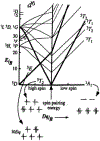
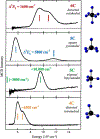
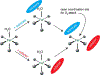
Non-heme iron enzymes play key roles in antibiotic, neurotransmitter, and natural product biosynthesis, DNA repair, hypoxia regulation, and disease states. These enzymes had been refractory to traditional bioinorganic spectroscopic methods. Thus, we developed variable-temperature variable-field magnetic circular dichroism (VTVH MCD) spectroscopy to experimentally define the excited and ground ligand field states of non-heme ferrous enzymes (Solomon et al., 1995). This method provides detailed geometric and electronic structure insight and thus enables a molecular level understanding of catalytic mechanisms. Application of this method across the five classes of non-heme ferrous enzymes has defined that a general mechanistic strategy is utilized where O2 activation is controlled to occur only in the presence of all cosubstrates.
Keywords: Ferrous enzyme mechanisms; Ligand field theory; Magnetic circular dichroism spectroscopy; Non-Kramers’ ions; Non-heme iron enzymes; Saturation magnetization.
Copyright © 2024. Published by Elsevier Inc.
Figures









Similar articles
-
Invited award contribution for ACS Award in Inorganic Chemistry. Geometric and electronic structure contributions to function in bioinorganic chemistry: active sites in non-heme iron enzymes.Inorg Chem. 2001 Jul 16;40(15):3656-69. doi: 10.1021/ic010348a. Inorg Chem. 2001. PMID: 11442362
-
Geometric and electronic structure contributions to function in non-heme iron enzymes.Acc Chem Res. 2013 Nov 19;46(11):2725-39. doi: 10.1021/ar400149m. Epub 2013 Sep 26. Acc Chem Res. 2013. PMID: 24070107 Free PMC article.
-
Spectroscopic and quantum chemical characterization of the electronic structure and bonding in a non-heme FeIV[double bond]O complex.J Am Chem Soc. 2004 May 5;126(17):5378-9. doi: 10.1021/ja0498033. J Am Chem Soc. 2004. PMID: 15113207
-
Stalking intermediates in oxygen activation by iron enzymes: motivation and method.J Inorg Biochem. 2006 Apr;100(4):586-605. doi: 10.1016/j.jinorgbio.2006.01.022. Epub 2006 Mar 2. J Inorg Biochem. 2006. PMID: 16513177 Review.
-
Structure/function correlations over binuclear non-heme iron active sites.J Biol Inorg Chem. 2016 Sep;21(5-6):575-88. doi: 10.1007/s00775-016-1372-9. Epub 2016 Jul 1. J Biol Inorg Chem. 2016. PMID: 27369780 Free PMC article. Review.
References
-
- Babicz JT, Rogers MS, DeWeese DE, Sutherlin KD, Banerjee R, Böttger LH, Yoda Y, Nagasawa N, Saito M, Kitao S, Kurokuzu M, Kobayashi Y, Tamasaku K, Seto M, Lipscomb JD, & Solomon EI (2023). Nuclear Resonance Vibrational Spectroscopy Definition of Peroxy Intermediates in Catechol Dioxygenases: Factors that Determine Extra-versus Intradiol Cleavage. Journal of the American Chemical Society. 10.1021/JACS.3C02242 - DOI - PMC - PubMed
-
- Buckingham AD, & Stephens PJ (1966). Magnetic Optical Activity. Annual Review of Physical Chemistry, 17(1), 399–432. 10.1146/annurev.pc.17.100166.002151 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

