High sugar diet promotes tumor progression paradoxically through aberrant upregulation of pepck1
- PMID: 39261338
- PMCID: PMC11390995
- DOI: 10.1007/s00018-024-05438-2
High sugar diet promotes tumor progression paradoxically through aberrant upregulation of pepck1
Abstract
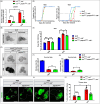
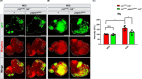
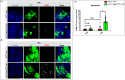
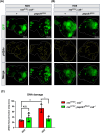
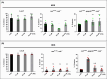
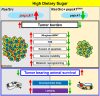
High dietary sugar (HDS), a contemporary dietary concern due to excessive intake of added sugars and carbohydrates, escalates the risk of metabolic disorders and concomitant cancers. However, the molecular mechanisms underlying HDS-induced cancer progression are not completely understood. We found that phosphoenolpyruvate carboxykinase 1 (PEPCK1), a pivotal enzyme in gluconeogenesis, is paradoxically upregulated in tumors by HDS, but not by normal dietary sugar (NDS), during tumor progression. Targeted knockdown of pepck1, but not pepck2, specifically in tumor tissue in Drosophila in vivo, not only attenuates HDS-induced tumor growth but also significantly improves the survival of Ras/Src tumor-bearing animals fed HDS. Interestingly, HP1a-mediated heterochromatin interacts directly with the pepck1 gene and downregulates pepck1 gene expression in wild-type Drosophila. Mechanistically, we demonstrated that, under HDS conditions, pepck1 knockdown reduces both wingless and TOR signaling, decreases evasion of apoptosis, reduces genome instability, and suppresses glucose uptake and trehalose levels in tumor cells in vivo. Moreover, rational pharmacological inhibition of PEPCK1, using hydrazinium sulfate, greatly improves the survival of tumor-bearing animals with pepck1 knockdown under HDS. This study is the first to show that elevated levels of dietary sugar induce aberrant upregulation of PEPCK1, which promotes tumor progression through altered cell signaling, evasion of apoptosis, genome instability, and reprogramming of carbohydrate metabolism. These findings contribute to our understanding of the complex relationship between diet and cancer at the molecular, cellular, and organismal levels and reveal PEPCK1 as a potential target for the prevention and treatment of cancers associated with metabolic disorders.
Keywords: Cancer metabolism; Epigenetics; Gene regulation; Hallmarks of cancer; Model organisms; Signal transduction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- MOST 103-2320-B-006-022-MY3/Ministry of Science and Technology, Taiwan
- MOST 106-2320-B-006-041/Ministry of Science and Technology, Taiwan
- MOST 108-2320-B-006-033-MY3/Ministry of Science and Technology, Taiwan
- 111-2320-B-006-021-MY3/Ministry of Science and Technology, Taiwan
- 112-2811-B-006-047/Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

