A Peptide Motif Covering Splice Site B in Neuroligin-1 Binds to Aβ and Acts as a Neprilysin Inhibitor
- PMID: 39261388
- PMCID: PMC11790763
- DOI: 10.1007/s12035-024-04475-z
A Peptide Motif Covering Splice Site B in Neuroligin-1 Binds to Aβ and Acts as a Neprilysin Inhibitor
Abstract
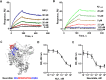
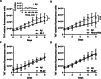
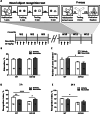
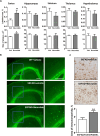
The most common cause of dementia among elderly people is Alzheimer's disease (AD). The typical symptom of AD is the decline of cognitive abilities, which is caused by loss of synaptic function. Amyloid-β (Aβ) oligomers play a significant role in the development of this synaptic dysfunction. Neuroligin-(NL)1 is a postsynaptic cell-adhesion molecule located in excitatory synapses and involved in the maintenance and modulation of synaptic contacts. A recent study has found that Aβ interacts with the soluble N-terminal fragment of NL1. The present study aimed to elucidate the role of NL1 in Aβ-induced neuropathology. Employing surface plasmon resonance and competitive ELISA, we confirmed the high-affinity binding of NL1 to the Aβ peptide. We also identified a sequence motif representing the NL1-binding site for the Aβ peptide and showed that a synthetic peptide modeled after this motif, termed neurolide, binds to the Aβ peptide with high affinity, comparable to the NL1-Aβ interaction. To assess the effect of neurolide in vivo, wild-type and 5XFAD mice were subcutaneously treated with this peptide for 10 weeks. We observed an increase in Aβ plaque formation in the cortex of neurolide-treated 5XFAD mice. Furthermore, we showed that neurolide reduces the activity of neprilysin, the predominant Aβ-degrading enzyme in the brain. Accordingly, we suggest that neurolide is the NL1-binding site for Aβ peptide, and acts as an inhibitor of neprilysin activity. Based on these data, we confirm the involvement of NL1 in the development of AD and suggest a mechanism for NL1-induced Aβ plaque formation.
Keywords: Alzheimer’s disease; Amyloid-β; Neprilysin; Neuroligin-1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: All animal experiments were performed in accordance to European Union law with licenses from the Danish Animal Experiments Inspectorate and from the Ethical Committee of the Estonian Ministry of Agriculture. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures
Similar articles
-
The synaptic protein neuroligin-1 interacts with the amyloid β-peptide. Is there a role in Alzheimer's disease?Biochemistry. 2011 Sep 27;50(38):8127-37. doi: 10.1021/bi201246t. Epub 2011 Sep 1. Biochemistry. 2011. PMID: 21838267
-
Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice.J Biol Chem. 2017 May 5;292(18):7327-7337. doi: 10.1074/jbc.M116.761189. Epub 2017 Mar 10. J Biol Chem. 2017. PMID: 28283575 Free PMC article.
-
The soluble extracellular fragment of neuroligin-1 targets Aβ oligomers to the postsynaptic region of excitatory synapses.Biochem Biophys Res Commun. 2015 Oct 9;466(1):66-71. doi: 10.1016/j.bbrc.2015.08.107. Epub 2015 Aug 29. Biochem Biophys Res Commun. 2015. PMID: 26325471
-
Understanding molecular mechanisms of proteolysis in Alzheimer's disease: progress toward therapeutic interventions.Biochim Biophys Acta. 2005 Aug 1;1751(1):60-7. doi: 10.1016/j.bbapap.2005.02.013. Epub 2005 Mar 17. Biochim Biophys Acta. 2005. PMID: 16054018 Review.
-
Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid.Rev Neurosci. 2009;20(1):1-12. doi: 10.1515/revneuro.2009.20.1.1. Rev Neurosci. 2009. PMID: 19526730 Review.
References
-
- Pozueta J, Lefort R, Shelanski ML (2013) Synaptic changes in Alzheimer’s disease and its models. Neuroscience 251:51–65. 10.1016/j.neuroscience.2012.05.050 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

