Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease
- PMID: 39261453
- PMCID: PMC11390906
- DOI: 10.1038/s41467-024-52214-8
Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease
Abstract
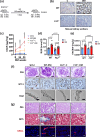
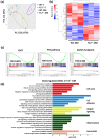
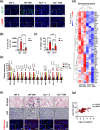
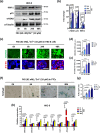
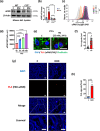
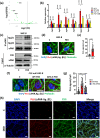
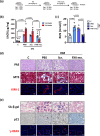
Coagulation factor XII (FXII) conveys various functions as an active protease that promotes thrombosis and inflammation, and as a zymogen via surface receptors like urokinase-type plasminogen activator receptor (uPAR). While plasma levels of FXII are increased in diabetes mellitus and diabetic kidney disease (DKD), a pathogenic role of FXII in DKD remains unknown. Here we show that FXII is locally expressed in kidney tubular cells and that urinary FXII correlates with kidney dysfunction in DKD patients. F12-deficient mice (F12-/-) are protected from hyperglycemia-induced kidney injury. Mechanistically, FXII interacts with uPAR on tubular cells promoting integrin β1-dependent signaling. This signaling axis induces oxidative stress, persistent DNA damage and senescence. Blocking uPAR or integrin β1 ameliorates FXII-induced tubular cell injury. Our findings demonstrate that FXII-uPAR-integrin β1 signaling on tubular cells drives senescence. These findings imply previously undescribed diagnostic and therapeutic approaches to detect or treat DKD and possibly other senescence-associated diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

