IL-1β promotes adipogenesis by directly targeting adipocyte precursors
- PMID: 39261467
- PMCID: PMC11390900
- DOI: 10.1038/s41467-024-51938-x
IL-1β promotes adipogenesis by directly targeting adipocyte precursors
Abstract
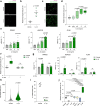
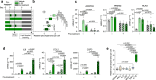
Postprandial IL-1β surges are predominant in the white adipose tissue (WAT), but its consequences are unknown. Here, we investigate the role of IL-1β in WAT energy storage and show that adipocyte-specific deletion of IL-1 receptor 1 (IL1R1) has no metabolic consequences, whereas ubiquitous lack of IL1R1 reduces body weight, WAT mass, and adipocyte formation in mice. Among all major WAT-resident cell types, progenitors express the highest IL1R1 levels. In vitro, IL-1β potently promotes adipogenesis in murine and human adipose-derived stem cells. This effect is exclusive to early-differentiation-stage cells, in which the adipogenic transcription factors C/EBPδ and C/EBPβ are rapidly upregulated by IL-1β and enriched near important adipogenic genes. The pro-adipogenic, but not pro-inflammatory effect of IL-1β is potentiated by acute treatment and blocked by chronic exposure. Thus, we propose that transient postprandial IL-1β surges regulate WAT remodeling by promoting adipogenesis, whereas chronically elevated IL-1β levels in obesity blunts this physiological function.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

