Symptom burden, coagulopathy and heart disease after acute SARS-CoV-2 infection in primary practice
- PMID: 39261512
- PMCID: PMC11390729
- DOI: 10.1038/s41598-024-71535-8
Symptom burden, coagulopathy and heart disease after acute SARS-CoV-2 infection in primary practice
Abstract
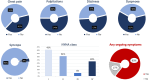
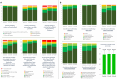
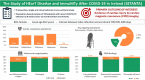
SETANTA (Study of HEarT DiseAse and ImmuNiTy After COVID-19 in Ireland) study aimed to investigate symptom burden and incidence of cardiac abnormalities after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 and to correlate these results with biomarkers of immunological response and coagulation. SETANTA was a prospective, single-arm observational cross-sectional study condcuted in a primary practice setting, and prospectively registered with ClinicalTrials.gov (identifier: NCT04823182). Patients with recent COVID-19 infection (≥ 6 weeks and ≤ 12 months) were prospectively enrolled. Primary outcomes of interest were markers of cardiac injury detected by cardiac magnetic resonance imaging (CMR), which included left ventricular ejection fraction, late gadolinium enhancement and pericardial abnormalities, as well as relevant biomarkers testing immunological response and coagulopathy. 100 patients (n = 129 approached) were included, amongst which 64% were female. Mean age of the total cohort was 45.2 years. The median (interquartile range) time interval between COVID-19 infection and enrolment was 189 [125, 246] days. 83% of participants had at least one persistent symptom, while 96% had positive serology for prior SARS-CoV-2 infection. Late gadolinium enhancement, pericardial effusion, was present in 2.2% and 8.3% respectively, while left ventricular ejection fraction was below the normal reference limit in 17.4% of patients. Von Willebrand factor antigen was elevated in 32.7% of patients and Fibrinogen and D-Dimer levels were found to be elevated in 10.2% and 11.1% of patients, respectively. In a cohort of primary practice patients recently recovered from SARS-CoV-2 infection, prevalence of persistent symptoms and markers of abnormal coagulation were high, despite a lower frequency of abnormalities on CMR compared with prior reports of patients assessed in a hospital setting.Trial Registration: Clinicaltrials.gov, NCT04823182 (prospectively registered on 30th March 2021).
Keywords: Biomarkers; COVID-19; Inflammation; Magnetic resonance imaging.
© 2024. The Author(s).
Conflict of interest statement
This study was funded by a Women As One Escalator Research Award 2021, awarded to the lead author, Dr. R. Colleran. R.A. Byrne reports research or educational funding to the institution of current employment from Abbott Vascular, Biosensors, Boston Scientific and Translumina; none of the funding contributes to his personal remuneration. L. McGovern is funded by the European Commission under Horizons 2020 framework (Grant agreement number 965246—CORE-MD). The other authors declare no potential conflicts of interest.
Figures
Similar articles
-
Cardiac pathology 6 months after hospitalization for COVID-19 and association with the acute disease severity.Am Heart J. 2021 Dec;242:61-70. doi: 10.1016/j.ahj.2021.08.001. Epub 2021 Aug 13. Am Heart J. 2021. PMID: 34400140 Free PMC article.
-
Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19).JAMA Cardiol. 2020 Nov 1;5(11):1265-1273. doi: 10.1001/jamacardio.2020.3557. JAMA Cardiol. 2020. PMID: 32730619 Free PMC article.
-
Cardiac sequelae after coronavirus disease 2019 recovery: a systematic review.Clin Microbiol Infect. 2021 Sep;27(9):1250-1261. doi: 10.1016/j.cmi.2021.06.015. Epub 2021 Jun 23. Clin Microbiol Infect. 2021. PMID: 34171458 Free PMC article.
-
Thromboelastography clot strength profiles and effect of systemic anticoagulation in COVID-19 acute respiratory distress syndrome: a prospective, observational study.Eur Rev Med Pharmacol Sci. 2020 Dec;24(23):12466-12479. doi: 10.26355/eurrev_202012_24043. Eur Rev Med Pharmacol Sci. 2020. PMID: 33336766
-
Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship.World J Gastroenterol. 2022 Mar 21;28(11):1102-1112. doi: 10.3748/wjg.v28.i11.1102. World J Gastroenterol. 2022. PMID: 35431501 Free PMC article. Review.
References
-
- Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1-epidemiology, pathophysiology, and diagnosis. Eur. Heart J.43(11), 1033–1058. 10.1093/eurheartj/ehab696 (2022). 10.1093/eurheartj/ehab696 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

