Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency
- PMID: 39261628
- PMCID: PMC12282641
- DOI: 10.1038/s42255-024-01121-9
Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency
Abstract
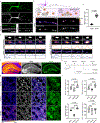
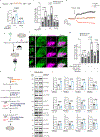
Glucose, the primary cellular energy source, is metabolized through glycolysis initiated by the rate-limiting enzyme hexokinase (HK). In energy-demanding tissues like the brain, HK1 is the dominant isoform, primarily localized on mitochondria, and is crucial for efficient glycolysis-oxidative phosphorylation coupling and optimal energy generation. This study unveils a unique mechanism regulating HK1 activity, glycolysis and the dynamics of mitochondrial coupling, mediated by the metabolic sensor enzyme O-GlcNAc transferase (OGT). OGT catalyses reversible O-GlcNAcylation, a post-translational modification influenced by glucose flux. Elevated OGT activity induces dynamic O-GlcNAcylation of the regulatory domain of HK1, subsequently promoting the assembly of the glycolytic metabolon on the outer mitochondrial membrane. This modification enhances the mitochondrial association with HK1, orchestrating glycolytic and mitochondrial ATP production. Mutation in HK1's O-GlcNAcylation site reduces ATP generation in multiple cell types, specifically affecting metabolic efficiency in neurons. This study reveals a previously unappreciated pathway that links neuronal metabolism and mitochondrial function through OGT and the formation of the glycolytic metabolon, providing potential strategies for tackling metabolic and neurological disorders.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures













References
MeSH terms
Substances
Grants and funding
- 5T32NS007220/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 7555.04/Gordon and Betty Moore Foundation (Gordon E. and Betty I. Moore Foundation)
- 5T32GM133351/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32 EB009380/EB/NIBIB NIH HHS/United States
- R35 GM128823/GM/NIGMS NIH HHS/United States
- T32 GM133351/GM/NIGMS NIH HHS/United States
- 5T32NS007220-40/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 2020-222005/Silicon Valley Community Foundation (SVCF)
- 2020298734/National Science Foundation (NSF)
- K12 GM068524/GM/NIGMS NIH HHS/United States
- MCB-1942763/National Science Foundation (NSF)
- T32 GM007240/GM/NIGMS NIH HHS/United States
- 5T32EB009380-15/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K12GM068524/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 NS094219/NS/NINDS NIH HHS/United States
- R35GM128823/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 2T32GM007240/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01NS094219/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- T32 NS007220/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

