Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium
- PMID: 39261670
- PMCID: PMC11390883
- DOI: 10.1038/s41598-024-72262-w
Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium
Abstract
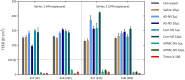
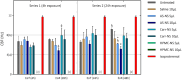
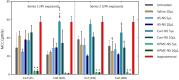
COVID-19 remains a severe condition for many including immunocompromised individuals. There remains a need for effective measures against this and other respiratory infections, which transmit via virus-laden droplets that reach the nasal or oral mucosae. Nasal sprays offer potential protection against viruses. Such formulations should preserve normal nasal mucociliary function. The antiviral barrier efficacy and effects on mucociliary function of astodrimer sodium nasal spray (AS-NS) were evaluated and compared with other available nasal sprays-low pH hydroxypropyl methylcellulose (HPMC-NS), iota-carrageenan (Carr-NS), nitric oxide (NO-NS), and povidone iodine (PI-NS). Assays simulated clinical conditions. Antiviral barrier function and cell viability were assessed in airway cell monolayers, while a model of fully differentiated human nasal epithelium (MucilAir™) was utilized to evaluate tissue integrity, cytotoxicity, cilia beating frequency, and mucociliary clearance. AS-NS reduced infectious virus in cell monolayers and demonstrated a benign cytotoxicity profile. In human nasal epithelium ex vivo, AS-NS had no impact on mucociliary function (cilia beating nor mucociliary clearance). Carr-NS, HPMC-NS, NO-NS and PI-NS demonstrated limited antiviral effects, while HPMC-NS caused inhibition of mucociliary function. Astodrimer sodium nasal spray demonstrates an acceptable nonclinical efficacy and safety profile as a barrier nasal spray against respiratory viral infection in the nasal cavity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential conflicts of interest: J.R.A.P., A.S. and G.P.H. are paid employees of Starpharma Pty Ltd. A.C. and C.A.L. are paid consultants to Starpharma Pty Ltd. S.C. is a paid employee of Epithelix Sarl. The remaining authors M.B. and P.A.G. declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

