ISB 2001 trispecific T cell engager shows strong tumor cytotoxicity and overcomes immune escape mechanisms of multiple myeloma cells
- PMID: 39261676
- PMCID: PMC11505469
- DOI: 10.1038/s43018-024-00821-1
ISB 2001 trispecific T cell engager shows strong tumor cytotoxicity and overcomes immune escape mechanisms of multiple myeloma cells
Abstract
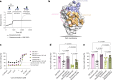
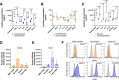
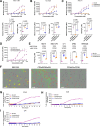
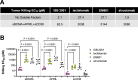
Despite recent advances in immunotherapies targeting single tumor-associated antigens, patients with multiple myeloma eventually relapse. ISB 2001 is a CD3+ T cell engager (TCE) co-targeting BCMA and CD38 designed to improve cytotoxicity against multiple myeloma. Targeting of two tumor-associated antigens by a single TCE resulted in superior cytotoxic potency across a variable range of BCMA and CD38 tumor expression profiles mimicking natural tumor heterogeneity, improved resistance to competing soluble factors and exhibited superior cytotoxic potency on patient-derived samples and in mouse models. Despite the broad expression of CD38 across human tissues, ISB 2001 demonstrated a reduced T cell activation profile in the absence of tumor cells when compared to TCEs targeting CD38 only. To determine an optimal first-in-human dose for the ongoing clinical trial ( NCT05862012 ), we developed an innovative quantitative systems pharmacology model leveraging preclinical data, using a minimum pharmacologically active dose approach, therefore reducing patient exposure to subefficacious doses of therapies.
© 2024. The Author(s).
Conflict of interest statement
L.C.-I., O.J.H., J.B., D.P., A.D., C.E., C.D., T. Monney, J.M., M.C., J.L., C.P., V.M., A.L., I.G., L.N.C., G.S.G., S.B., M.L.M., R.C.-W., A.S., M.D., E.A.Z., C.K., M. Perro and M. Pihlgren are employees or former employees of Ichnos Sciences or Ichnos Glenmark Innovation. T. Matsuura and P.H.v.d.G. are employees of Certara UK. P. Moreau is a member of the scientific advisory board of Ichnos Glenmark Innovation. T. Matthes, Z.K., C.M.E., J.R.E., S.M., C.P.-D. and C.T. declare no competing interests.
Figures











References
-
- Usmani, S. Z. et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol.7, e447–e455 (2020). - PubMed
-
- Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med.384, 705–716 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

