Green synthesis of polyethylene glycol coated, ciprofloxacin loaded CuO nanoparticles and its antibacterial activity against Staphylococcus aureus
- PMID: 39261712
- PMCID: PMC11390890
- DOI: 10.1038/s41598-024-72322-1
Green synthesis of polyethylene glycol coated, ciprofloxacin loaded CuO nanoparticles and its antibacterial activity against Staphylococcus aureus
Abstract
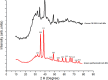
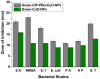
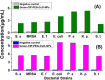
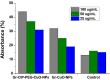
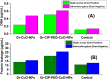
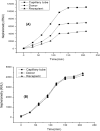
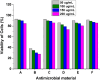
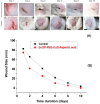
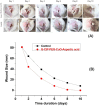
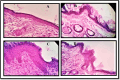
Antibacterial resistance requires an advanced strategy to increase the efficacy of current therapeutics in addition to the synthesis of new generations of antibiotics. In this study, copper oxide nanoparticles (CuO-NPs) were green synthesized using Moringa oleifera root extract. CuO-NPs fabricated into a form of aspartic acid-ciprofloxacin-polyethylene glycol coated copper oxide-nanotherapeutics (CIP-PEG-CuO) to improve the antibacterial activity of NPs and the efficacy of the drug with controlled cytotoxicity. These NPs were charachterized by Fourier transform infrared spectroscopy (FTIR), x-rays diffraction spectroscopy (XRD), scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS). Antibacterial screening and bacterial chemotaxis investigations demonstrated that CIP-PEG-CuO NPs show enhanced antibacterial potential against Gram-positive and Gram-negative clinically isolated pathogenic bacterial strains as compared to CuO-NPs. In ex-vivo cytotoxicity CIP-PEG-CuO-nano-formulates revealed 88% viability of Baby Hamster Kidney 21 cell lines and 90% RBCs remained intact with nano-formulations during hemolysis assay. An in-vivo studies on animal models show that Staphylococcus aureus were eradicated by this newly developed formulate from the infected skin and showed wound-healing properties. By using specially designed nanoparticles that are engineered to precisely transport antimicrobial agents, these efficient nano-drug delivery systems can target localized infections, ensure targeted delivery, enhance efficacy through increased drug penetration through physical barriers, and reduce systemic side effects for more effective treatment.
Keywords: Staphylococcus aureus; Antibacterial; CIP-PEG-CuO-nanotherapeutic; Green synthesis; Nano-drug delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Ciprofloxacin loaded PEG coated ZnO nanoparticles with enhanced antibacterial and wound healing effects.Sci Rep. 2024 Feb 26;14(1):4689. doi: 10.1038/s41598-024-55306-z. Sci Rep. 2024. PMID: 38409460 Free PMC article.
-
Green synthesis of copper oxide nanoparticles using Abutilon indicum leaves extract and their evaluation of antibacterial, anticancer in human A549 lung and MDA-MB-231 breast cancer cells.Food Chem Toxicol. 2022 Oct;168:113330. doi: 10.1016/j.fct.2022.113330. Epub 2022 Aug 1. Food Chem Toxicol. 2022. PMID: 35926645
-
An Eco-Friendly Synthesis Approach for Enhanced Photocatalytic and Antibacterial Properties of Copper Oxide Nanoparticles Using Coelastrella terrestris Algal Extract.Int J Nanomedicine. 2024 May 9;19:4137-4162. doi: 10.2147/IJN.S452889. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38756417 Free PMC article.
-
Eco-friendly copper nanoparticles embedded cellulose aerogel from corn husk with robust antibacterial and catalytic reduction performance.Int J Biol Macromol. 2025 May;310(Pt 2):143359. doi: 10.1016/j.ijbiomac.2025.143359. Epub 2025 Apr 19. Int J Biol Macromol. 2025. PMID: 40258545 Review.
-
Antibacterial Properties of Copper Oxide Nanoparticles (Review).Int J Mol Sci. 2024 Oct 28;25(21):11563. doi: 10.3390/ijms252111563. Int J Mol Sci. 2024. PMID: 39519117 Free PMC article. Review.
Cited by
-
Plant extract-mediated green-synthesized CuO nanoparticles for environmental and microbial remediation: a review covering basic understandings to mechanistic study.Nanoscale Adv. 2025 Mar 19;7(9):2418-2445. doi: 10.1039/d5na00035a. eCollection 2025 Apr 29. Nanoscale Adv. 2025. PMID: 40207087 Free PMC article. Review.
-
Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings.Gels. 2025 Feb 7;11(2):123. doi: 10.3390/gels11020123. Gels. 2025. PMID: 39996666 Free PMC article. Review.
References
-
- Church, N. A. & McKillip, J. L. Antibiotic resistance crisis: Challenges and imperatives. Biologia76, 1535–1550 (2021).10.1007/s11756-021-00697-x - DOI
-
- Türkyılmaz, O. & Darcan, C. The emergence and preventability of globally spreading antibiotic resistance: A literature review. Biol. Bullet. Rev.13, 578–589 (2023).10.1134/S2079086423060154 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

