Agonist antibody to guanylate cyclase receptor NPR1 regulates vascular tone
- PMID: 39261724
- PMCID: PMC11410649
- DOI: 10.1038/s41586-024-07903-1
Agonist antibody to guanylate cyclase receptor NPR1 regulates vascular tone
Abstract
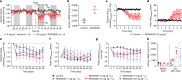
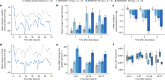
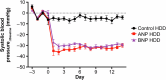
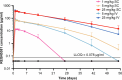
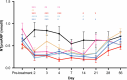
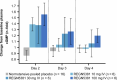
Heart failure is a leading cause of morbidity and mortality1,2. Elevated intracardiac pressures and myocyte stretch in heart failure trigger the release of counter-regulatory natriuretic peptides, which act through their receptor (NPR1) to affect vasodilation, diuresis and natriuresis, lowering venous pressures and relieving venous congestion3-8. Recombinant natriuretic peptide infusions were developed to treat heart failure but have been limited by a short duration of effect9,10. Here we report that in a human genetic analysis of over 700,000 individuals, lifelong exposure to coding variants of the NPR1 gene is associated with changes in blood pressure and risk of heart failure. We describe the development of REGN5381, an investigational monoclonal agonist antibody that targets the membrane-bound guanylate cyclase receptor NPR1. REGN5381, an allosteric agonist of NPR1, induces an active-like receptor conformation that results in haemodynamic effects preferentially on venous vasculature, including reductions in systolic blood pressure and venous pressure in animal models. In healthy human volunteers, REGN5381 produced the expected haemodynamic effects, reflecting reductions in venous pressures, without obvious changes in diuresis and natriuresis. These data support the development of REGN5381 for long-lasting and selective lowering of venous pressures that drive symptomatology in patients with heart failure.
© 2024. The Author(s).
Conflict of interest statement
M.E.D., A.K., J.H.K., A.J.-H.H., M.C.F., X.J., D.Z., J.T., E.G., N.L., M.R., H.P., S.K., M.E.B., W.Z., A.R., J.B.N., T.D., N.V., A.P., A.L., A.B., L.A.L., B.J.M., J. Mastaitis, K.B.D.-N., T.H., G.H., W.O., A.J.M., G.D.Y., B.A.O. and L.M. are employees of and shareholders in Regeneron Pharmaceuticals. A.R.H., J. Meyer, D.J., V.K. and A.J.R. were employees of Regeneron Pharmaceuticals at the time of the studies. The other authors declare no competing interests.
Figures








Comment in
-
Agonist antibody lowers blood pressure.Nat Rev Drug Discov. 2024 Nov;23(11):815. doi: 10.1038/d41573-024-00151-y. Nat Rev Drug Discov. 2024. PMID: 39313597 No abstract available.
References
-
- Lippi, G. & Sanchis-Gomar, F. Global epidemiology and future trends of heart failure. AME Med. J.5, 15 (2020).
-
- Ledsome, J. R., Wilson, N., Courneya, C. A. & Rankin, A. J. Release of atrial natriuretic peptide by atrial distension. Can. J. Physiol. Pharmacol.63, 739–742 (1985). - PubMed
-
- Levin, E. R., Gardner, D. G. & Samson, W. K. Natriuretic peptides. N. Engl. J. Med.339, 321–328 (1998). - PubMed
-
- Ramos, H. & de Bold, A. J. Gene expression, processing, and secretion of natriuretic peptides: physiologic and diagnostic implications. Heart Fail. Clin.2, 255–268 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

