Brain-wide dynamics linking sensation to action during decision-making
- PMID: 39261727
- PMCID: PMC11499283
- DOI: 10.1038/s41586-024-07908-w
Brain-wide dynamics linking sensation to action during decision-making
Abstract
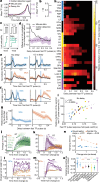
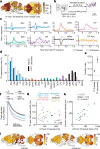
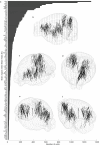
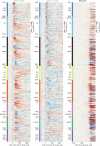
Perceptual decisions rely on learned associations between sensory evidence and appropriate actions, involving the filtering and integration of relevant inputs to prepare and execute timely responses1,2. Despite the distributed nature of task-relevant representations3-10, it remains unclear how transformations between sensory input, evidence integration, motor planning and execution are orchestrated across brain areas and dimensions of neural activity. Here we addressed this question by recording brain-wide neural activity in mice learning to report changes in ambiguous visual input. After learning, evidence integration emerged across most brain areas in sparse neural populations that drive movement-preparatory activity. Visual responses evolved from transient activations in sensory areas to sustained representations in frontal-motor cortex, thalamus, basal ganglia, midbrain and cerebellum, enabling parallel evidence accumulation. In areas that accumulate evidence, shared population activity patterns encode visual evidence and movement preparation, distinct from movement-execution dynamics. Activity in movement-preparatory subspace is driven by neurons integrating evidence, which collapses at movement onset, allowing the integration process to reset. Across premotor regions, evidence-integration timescales were independent of intrinsic regional dynamics, and thus depended on task experience. In summary, learning aligns evidence accumulation to action preparation in activity dynamics across dozens of brain regions. This leads to highly distributed and parallelized sensorimotor transformations during decision-making. Our work unifies concepts from decision-making and motor control fields into a brain-wide framework for understanding how sensory evidence controls actions.
© 2024. Crown.
Conflict of interest statement
The authors declare no competing interests
Figures














References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

