Drosophila are hosts to the first described parasitoid wasp of adult flies
- PMID: 39261731
- PMCID: PMC11424482
- DOI: 10.1038/s41586-024-07919-7
Drosophila are hosts to the first described parasitoid wasp of adult flies
Abstract
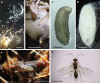
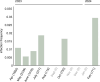
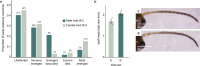
Parasitoid wasps are exceptionally diverse and use specialized adaptations capable of manipulating the physiology and behaviour of host organisms1. In more than two centuries since the first records of Drosophila-parasitizing wasps, nearly 200 described and provisional parasitoid species of drosophilids have been identified2. These include endoparasitoids and ectoparasitoids, as well as species attacking larval and pupal hosts3. Despite a deep history of research attention and remarkable biodiversity, a wasp species that attacks and develops inside the adult stage of a fly host has not been described previously. Here we report the discovery of a wasp species that infects the adult stage of fruit flies in the genus Drosophila, including one of the most deeply studied model organisms in biology, Drosophila melanogaster. Notably, this wasp can be easily collected from backyard fly baits and has a broad geographic distribution throughout the eastern USA. We document its life history and unique host interactions, including egg-laying into and larval emergence from adult flies, and provide protocols to raise wasps from wild-caught host flies. Our results emphasize the need for ongoing research investment in insect biodiversity and systematics. As parasitoid research continues to uncover unusual biology and supports fundamental mechanistic insights into immunity4, metabolism5, ecology6, evolution7-9 and behaviour10-12, we anticipate that this wasp's association with the laboratory model organism, D. melanogaster, will provide new research opportunities across the life sciences.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Prevost, G. Parasitoids of Drosophila (Elsevier, 2009). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

