Self-organized tissue mechanics underlie embryonic regulation
- PMID: 39261736
- PMCID: PMC11424473
- DOI: 10.1038/s41586-024-07934-8
Self-organized tissue mechanics underlie embryonic regulation
Abstract
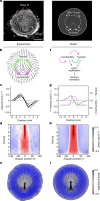
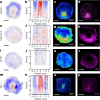
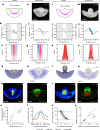
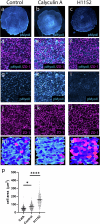
Early amniote development is highly self-organized, capable of adapting to interference through local and long-range cell-cell interactions. This process, called embryonic regulation1, has been well illustrated in experiments on avian embryos, in which subdividing the epiblast disk into different parts not only redirects cell fates to eventually form a complete and well-proportioned embryo at its original location, but also leads to the self-organization of additional, fully formed embryos2,3 in the other separated parts. The cellular interactions underlying embryonic self-organization are widely believed to be mediated by molecular signals, yet the identity of such signals is unclear. Here, by analysing intact and mechanically perturbed quail embryos, we show that the mechanical forces that drive embryogenesis self-organize, with contractility locally self-activating and the ensuing tension acting as a long-range inhibitor. This mechanical feedback governs the persistent pattern of tissue flows that shape the embryo4-6 and also steers the concomitant emergence of embryonic territories by modulating gene expression, ensuring the formation of a single embryo under normal conditions, yet allowing the emergence of multiple, well-proportioned embryos after perturbations. Thus, mechanical forces act at the core of embryonic self-organization, shaping both tissues and gene expression to robustly yet plastically canalize early development.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
A tension-induced morphological transition shapes the avian extra-embryonic territory.Curr Biol. 2025 Apr 21;35(8):1681-1692.e4. doi: 10.1016/j.cub.2025.02.028. Epub 2025 Mar 12. Curr Biol. 2025. PMID: 40081377
-
Mechanics of tissue compaction.Semin Cell Dev Biol. 2015 Dec;47-48:110-7. doi: 10.1016/j.semcdb.2015.08.001. Epub 2015 Aug 6. Semin Cell Dev Biol. 2015. PMID: 26256955 Free PMC article. Review.
-
Mechanical perspectives on the anterior-posterior axis polarization of mouse implanted embryos.Mech Dev. 2017 Apr;144(Pt A):62-70. doi: 10.1016/j.mod.2016.09.002. Epub 2016 Sep 30. Mech Dev. 2017. PMID: 27697519 Review.
-
Molecular architecture of lineage allocation and tissue organization in early mouse embryo.Nature. 2019 Aug;572(7770):528-532. doi: 10.1038/s41586-019-1469-8. Epub 2019 Aug 7. Nature. 2019. PMID: 31391582
-
Development: Turing mechanics.Curr Biol. 2024 Dec 16;34(24):R1230-R1232. doi: 10.1016/j.cub.2024.10.065. Curr Biol. 2024. PMID: 39689690
Cited by
-
Bilateral cellular flows display asymmetry prior to left-right organizer formation in amniote gastrulation.bioRxiv [Preprint]. 2024 Dec 29:2024.04.21.590437. doi: 10.1101/2024.04.21.590437. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2414860122. doi: 10.1073/pnas.2414860122. PMID: 38712212 Free PMC article. Updated. Preprint.
-
A chemo-mechanical model of endoderm movements driving elongation of the amniote hindgut.bioRxiv [Preprint]. 2023 May 18:2023.05.18.541363. doi: 10.1101/2023.05.18.541363. bioRxiv. 2023. Update in: Development. 2023 Nov 15;150(22):dev202010. doi: 10.1242/dev.202010. PMID: 37292966 Free PMC article. Updated. Preprint.
-
A mechanochemical model recapitulates distinct vertebrate gastrulation modes.Sci Adv. 2023 Dec 8;9(49):eadh8152. doi: 10.1126/sciadv.adh8152. Epub 2023 Dec 6. Sci Adv. 2023. PMID: 38055823 Free PMC article.
-
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos.Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2302879120. doi: 10.1073/pnas.2302879120. Epub 2023 Oct 25. Proc Natl Acad Sci U S A. 2023. PMID: 37878715 Free PMC article.
-
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos.bioRxiv [Preprint]. 2023 Mar 20:2023.03.16.532979. doi: 10.1101/2023.03.16.532979. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2302879120. doi: 10.1073/pnas.2302879120. PMID: 36993669 Free PMC article. Updated. Preprint.
References
-
- Driesch, H. The Science and Philosophy of the Organism the Gifford Lectures Delivered Before the University of Aberdeen in the Year 1907–1908 (Adam and Charles Black, 1908).
-
- Lutz, H. Sur la Production Expérimentale de la Polyembryonie et de la Monstruosité Double Chez les Oiseau (Université de Strasbourg, 1949).
-
- Spratt, N. T. & Haas, H. Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J. Exp. Zool.145, 97–137 (1960).
-
- Gräper, L. Die primitiventwicklung des hühnchens nach stereokinematographischen untersuchungen, kontrolliert durch vitale farbmarkierung und verglichen mit der entwicklung anderer wirbeltiere. Dev. Genes Evol.116, 382–429 (1929). - PubMed
-
- Wetzel, R. Untersuchungen am Hühnchen. Die entwicklung des keims während der ersten beiden bruttage. Wilhelm Roux Arch. Entwickl. Mech. Org.119, 188–321 (1929). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

