Multi-pass, single-molecule nanopore reading of long protein strands
- PMID: 39261738
- PMCID: PMC11410661
- DOI: 10.1038/s41586-024-07935-7
Multi-pass, single-molecule nanopore reading of long protein strands
Abstract
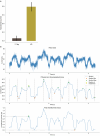
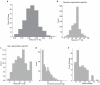
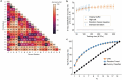
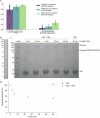
The ability to sequence single protein molecules in their native, full-length form would enable a more comprehensive understanding of proteomic diversity. Current technologies, however, are limited in achieving this goal1,2. Here, we establish a method for the long-range, single-molecule reading of intact protein strands on a commercial nanopore sensor array. By using the ClpX unfoldase to ratchet proteins through a CsgG nanopore3,4, we provide single-molecule evidence that ClpX translocates substrates in two-residue steps. This mechanism achieves sensitivity to single amino acids on synthetic protein strands hundreds of amino acids in length, enabling the sequencing of combinations of single-amino-acid substitutions and the mapping of post-translational modifications, such as phosphorylation. To enhance classification accuracy further, we demonstrate the ability to reread individual protein molecules multiple times, and we explore the potential for highly accurate protein barcode sequencing. Furthermore, we develop a biophysical model that can simulate raw nanopore signals a priori on the basis of residue volume and charge, enhancing the interpretation of raw signal data. Finally, we apply these methods to examine full-length, folded protein domains for complete end-to-end analysis. These results provide proof of concept for a platform that has the potential to identify and characterize full-length proteoforms at single-molecule resolution.
© 2024. The Author(s).
Conflict of interest statement
The University of Washington has filed provisional patent applications covering protein rereading (K.M. and J.N.) and sequence-to-signal simulation methods (D.K.-H., M.Q., and J.N.). J.N. is a consultant to Oxford Nanopore Technologies and holds share options in the company. The other authors declare no competing interests.
Figures
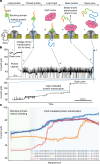
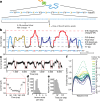
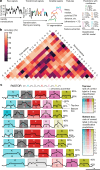



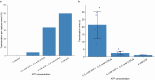





Update of
-
Multi-pass, single-molecule nanopore reading of long protein strands with single-amino acid sensitivity.bioRxiv [Preprint]. 2023 Oct 20:2023.10.19.563182. doi: 10.1101/2023.10.19.563182. bioRxiv. 2023. Update in: Nature. 2024 Sep;633(8030):662-669. doi: 10.1038/s41586-024-07935-7. PMID: 37905023 Free PMC article. Updated. Preprint.
References
-
- Dorey, A. & Howorka, S. Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics. Nat. Chem.16, 314–334 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

