Effects of the FHL2 gene on the development of subcutaneous and intramuscular adipocytes in goats
- PMID: 39261767
- PMCID: PMC11389066
- DOI: 10.1186/s12864-024-10755-8
Effects of the FHL2 gene on the development of subcutaneous and intramuscular adipocytes in goats
Erratum in
-
Correction: Effects of the FHL2 gene on the development of subcutaneous and intramuscular adipocytes in goats.BMC Genomics. 2025 Jun 17;26(1):570. doi: 10.1186/s12864-025-11789-2. BMC Genomics. 2025. PMID: 40528156 Free PMC article. No abstract available.
Abstract
Background: Adipose tissue affects not only the meat quality of domestic animals, but also human health. Adipocyte differentiation is regulated by a series of regulatory genes and cyclins. Four and half-LIM protein (FHL2) is positively correlated with the hypertrophy of adipocytes and can cause symptoms such as obesity and diabetes.
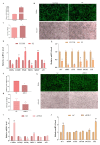
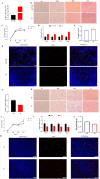
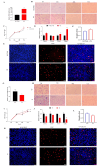
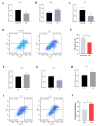
Result: In the transcriptome sequencing analysis of intramuscular adipocytes after three days of differentiation, the differentially expressed gene FHL2 was found. To further explore the biological significance of the differentially expressed gene FHL2, which was downregulated in the mature adipocytes. We revealed the function of FHL2 in adipogenesis through the acquisition and loss of function of FHL2. The results showed that the overexpression of FHL2 significantly increased the expression of adipogenic genes (PPARγ, C/EBPβ) and the differentiation of intramuscular and subcutaneous adipocytes. However, silencing FHL2 significantly inhibited adipocyte differentiation. The overexpression of FHL2 increased the number of adipocytes stained with crystal violet and increased the mRNA expression of proliferation marker genes such as CCNE, PCNA, CCND and CDK2. In addition, it significantly increased the rate of EdU positive cells. In terms of apoptosis, overexpression of FHL2 significantly inhibited the expression of P53 and BAX in both intramuscular and subcutaneous adipocytes, which are involved in cell apoptosis. However, overexpression of FHL2 promoted the expression of BCL, but was rescued by the silencing of FHL2.
Conclusions: In summary, FHL2 may be a positive regulator of intramuscular and subcutaneous adipocyte differentiation and proliferation, and acts as a negative regulator of intramuscular and subcutaneous adipocyte apoptosis. These findings provide a theoretical basis for the subsequent elucidation of FHL2 in adipocytes.
Keywords: FHL2; Adipogenesis; Goat; Intramuscular adipocytes; Subcutaneous adipocytes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

