M2 macrophage-derived exosomes enable osteogenic differentiation and inhibit inflammation in human periodontal ligament stem cells through promotion of CXCL12 expression
- PMID: 39261847
- PMCID: PMC11391719
- DOI: 10.1186/s12903-024-04831-4
M2 macrophage-derived exosomes enable osteogenic differentiation and inhibit inflammation in human periodontal ligament stem cells through promotion of CXCL12 expression
Abstract
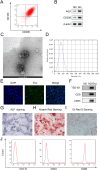
Background: Periodontitis is a dental disease characterized by inflammation of periodontal tissues and loss of the periodontal ligaments and alveolar bone. Exosomes are a class of extracellular vesicles that are involved in a variety of diseases by releasing active substances. In this study, we aimed to investigate the effect and mechanism of exosomes from M2 polarized macrophages (M2-exos) on osteogenic differentiation in human periodontal ligament stem cells (hPDLSCs).
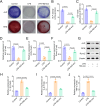
Methods: M2-exos were isolated from IL-4-induced RAW264.7 cells (M2 macrophages) and then treated on hPDLSCs. Osteogenic differentiation was assessed by alkaline phosphatase (ALP) staining, alizarin red S (ARS) staining, measurement of osteogenic differentiation-related genes and proteins, and inflammation was evaluated by measuring the levels of inflammatory factors. The mechanism of M2-exo was confirmed through qPCR, western blot, ALP and ARS staining.
Results: Results suggested that M2-exo improved osteogenic differentiation and inhibited inflammation in LPS-induced hPDLSCs. CXCL12 expression was elevated in M2 macrophages, but decreased in LPS-induced hPDLSCs. Moreover, the effect of M2-exo on osteogenic differentiation and inflammation in LPS-induced hPDLSCs was reversed by CXCL12 knockdown.
Conclusion: We demonstrated that M2-exo facilitated osteogenic differentiation and suppressed inflammation in LPS-induced hPDLSCs through promotion of CXCL12 expression. These results suggested the potential of M2-exo in the treatment of periodontitis, which may provide a new theoretical basis for M2-exo treatment of periodontitis.
Keywords: CXCL12; Exosome; Macrophage; Osteogenic differentiation; Periodontitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

