USP14 inhibition promotes DNA damage repair and represses ovarian granulosa cell senescence in premature ovarian insufficiency
- PMID: 39261935
- PMCID: PMC11389224
- DOI: 10.1186/s12967-024-05636-3
USP14 inhibition promotes DNA damage repair and represses ovarian granulosa cell senescence in premature ovarian insufficiency
Abstract
Background: Premature ovarian insufficiency (POI) is a condition characterized by a substantial decline or loss of ovarian function in women before the age of 40. However, the pathogenesis of POI remains to be further elucidated, and specific targeted drugs which could delay or reverse ovarian reserve decline are urgently needed. Abnormal DNA damage repair (DDR) and cell senescence in granulosa cells are pathogenic mechanisms of POI. Ubiquitin-specific protease 14 (USP14) is a key enzyme that regulates the deubiquitylation of DDR-related proteins, but whether USP14 participates in the pathogenesis of POI remains unclear.
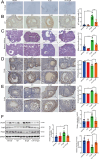
Methods: We measured USP14 mRNA expression in granulosa cells from biochemical POI (bPOI) patients. In KGN cells, we used IU1 and siRNA-USP14 to specifically inhibit USP14 and constructed a cell line stably overexpressing USP14 to examine its effects on DDR function and cellular senescence in granulosa cells. Next, we explored the therapeutic potential of IU1 in POI mouse models induced by D-galactose.
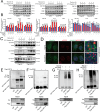
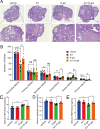
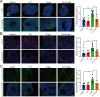
Results: USP14 expression in the granulosa cells of bPOI patients was significantly upregulated. In KGN cells, IU1 treatment and siUSP14 transfection decreased etoposide-induced DNA damage levels, promoted DDR function, and inhibited cell senescence. USP14 overexpression increased DNA damage, impaired DDR function, and promoted cell senescence. Moreover, IU1 treatment and siUSP14 transfection increased nonhomologous end joining (NHEJ), upregulated RNF168, Ku70, and DDB1, and increased ubiquitinated DDB1 levels in KGN cells. Conversely, USP14 overexpression had the opposite effects. Intraperitoneal IU1 injection alleviated etoposide-induced DNA damage in granulosa cells, ameliorated the D-galactose-induced POI phenotype, promoted DDR, and inhibited cell senescence in ovarian granulosa cells in vivo.
Conclusions: Upregulated USP14 in ovarian granulosa cells may play a role in POI pathogenesis, and targeting USP14 may be a potential POI treatment strategy. Our study provides new insights into the pathogenesis of POI and a novel POI treatment strategy.
Keywords: Cell senescence; DNA damage repair; Granulosa cells; Premature ovarian insufficiency; Ubiquitin-specific protease 14.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Wang X, Zhang X, Dang Y, Li D, Lu G, Chan WY, Leung PCK, Zhao S, Qin Y, Chen ZJ. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020;48(9):4480–91. 10.1093/nar/gkaa127 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

