Quinone chemistry in respiratory complex I involves protonation of a conserved aspartic acid residue
- PMID: 39262040
- PMCID: PMC11627005
- DOI: 10.1002/1873-3468.15013
Quinone chemistry in respiratory complex I involves protonation of a conserved aspartic acid residue
Abstract
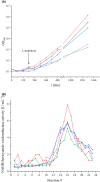
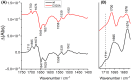
Respiratory complex I is a central metabolic enzyme coupling NADH oxidation and quinone reduction with proton translocation. Despite the knowledge of the structure of the complex, the coupling of both processes is not entirely understood. Here, we use a combination of site-directed mutagenesis, biochemical assays, and redox-induced FTIR spectroscopy to demonstrate that the quinone chemistry includes the protonation and deprotonation of a specific, conserved aspartic acid residue in the quinone binding site (D325 on subunit NuoCD in Escherichia coli). Our experimental data support a proposal derived from theoretical considerations that deprotonation of this residue is involved in triggering proton translocation in respiratory complex I.
Keywords: Escherichia coli; NADH dehydrogenase; NADH:quinone oxidoreductase; iron–sulfur cluster; proton‐coupled electron transfer; quinone reduction; redox‐induced FTIR spectroscopy; site‐directed mutagenesis.
© 2024 The Author(s). FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures

References
-
- Hirst J (2013) Mitochondrial complex I. Annu Rev Biochem 82, 551–575. - PubMed
-
- Friedrich T (2014) On the mechanism of respiratory complex I. J Bioenerg Biomembr 46, 255–269. - PubMed
-
- Sazanov LA (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16, 375–388. - PubMed
-
- Parey K, Wirth C, Vonck J and Zickermann V (2020) Respiratory complex I – structure, mechanism and evolution. Curr Opin Struct Biol 63, 1–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

