Unraveling the hydration dynamics of ACC1-13K24 with ATP: From liquid to droplet to amyloid fibril
- PMID: 39262114
- PMCID: PMC11617625
- DOI: 10.1016/j.bpj.2024.09.011
Unraveling the hydration dynamics of ACC1-13K24 with ATP: From liquid to droplet to amyloid fibril
Abstract
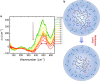
In order to achieve a comprehensive understanding of protein aggregation processes, an exploration of solvation dynamics, a key yet intricate component of biological phenomena, is mandatory. In the present study, we used Fourier transform infrared spectroscopy and terahertz spectroscopy complemented by atomic force microscopy and kinetic experiments utilizing thioflavin T fluorescence to elucidate the changes in solvation dynamics during liquid-liquid phase separation and subsequent amyloid fibril formation, the latter representing a transition from liquid to solid phase separation. These processes are pivotal in the pathology of neurodegenerative disorders such as Alzheimer's and Parkinson's diseases. We focus on the ACC1-13K24-ATP protein complex, which undergoes fibril formation followed by droplet generation. Our investigation reveals the importance of hydration as a driving force in these processes, offering new insights into the molecular mechanisms at play.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

