Identification of novel inhibitors of the transcriptional coactivator MRTF-A for HCC therapy
- PMID: 39262570
- PMCID: PMC11387234
- DOI: 10.1016/j.omton.2024.200855
Identification of novel inhibitors of the transcriptional coactivator MRTF-A for HCC therapy
Abstract
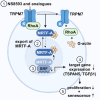
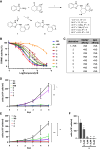
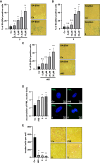
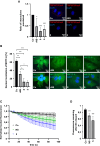
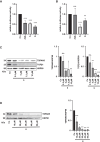
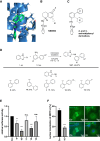
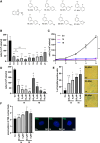
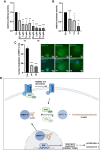
Myocardin-related transcription factor A (MRTF-A) is a coactivator of serum response factor (SRF), which regulates the expression of genes involved in cell proliferation, migration, and differentiation and has been implicated in hepatocellular carcinoma (HCC) progression. We recently established inhibition of the transcriptional activity of MRTF-A by NS8593 as a novel therapeutic approach for HCC therapy. NS8593 is a negative gating modulator of the transient receptor potential cation channel TRPM7. In this report, we identify an aminobenzimidazole that is highly potent in inhibiting TRPM7 and its interaction with RhoA, leading to decreased SRF transcriptional activity and enhanced nuclear export of MRTF-A, as determined by fluorescence loss in photobleaching (FLIP). This resulted in reduced expression of the MRTF/SRF target genes transforming growth factor β1 (TGF-β1) and tetraspanin 5 (TSPAN5), senescence induction, and growth arrest in HCC cells. Replacement of the tetraline core by a 3-aminophenyl substructure yielded inhibitor 10 with higher potency than inhibitor 5, and further structural modifications yielded highly potent inhibitors of SRF activity, 14 and 16. Both compounds were capable of inhibiting cell proliferation and inducing senescence in HCC cells with improved efficacy compared to NS8593. These inhibitors represent valuable tools for understanding the molecular basis of drug development targeting TRPM7 and MRTFs.
Keywords: HCC; MKL1; MRTF; MT: Regular Issue; NS8593; RhoA; SK; SRF; TGF-ß1; TRPM7; TSPAN5.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. - PubMed
-
- El-Serag H.B. Epidemiology of hepatocellular carcinoma in USA. Hepatol. Res. 2007;37:S88–S94. - PubMed
-
- Whittaker S., Marais R., Zhu A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. - PubMed
-
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. - PMC - PubMed
-
- Hampl V., Martin C., Aigner A., Hoebel S., Singer S., Frank N., Sarikas A., Ebert O., Prywes R., Gudermann T., Muehlich S. Depletion of the transcriptional coactivators megakaryoblastic leukaemia 1 and 2 abolishes hepatocellular carcinoma xenograft growth by inducing oncogene-induced senescence. EMBO Mol. Med. 2013;5:1367–1382. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
