Divergent iron regulatory states contribute to heterogeneity in breast cancer aggressiveness
- PMID: 39262774
- PMCID: PMC11387597
- DOI: 10.1016/j.isci.2024.110661
Divergent iron regulatory states contribute to heterogeneity in breast cancer aggressiveness
Abstract
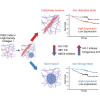
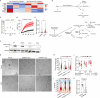
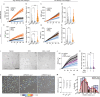
Contact with dense collagen I (Col1) can induce collective invasion of triple negative breast cancer (TNBC) cells and transcriptional signatures linked to poor patient prognosis. However, this response is heterogeneous and not well understood. Using phenotype-guided sequencing analysis of invasive vs. noninvasive subpopulations, we show that these two phenotypes represent opposite sides of the iron response protein 1 (IRP1)-mediated response to cytoplasmic labile iron pool (cLIP) levels. Invasive cells upregulate iron uptake and utilization machinery characteristic of a low cLIP response, which includes contractility regulating genes that drive migration. Non-invasive cells upregulate iron sequestration machinery characteristic of a high cLIP response, which is accompanied by upregulation of actin sequestration genes. These divergent IRP1 responses result from Col1-induced transient expression of heme oxygenase I (HO-1), which cleaves heme and releases iron. These findings lend insight into the emerging theory that heme and iron fluxes regulate TNBC aggressiveness.
Keywords: Cancer; Cell biology; Molecular biology; Molecular physiology.
© 2024 The Author(s).
Conflict of interest statement
S.I.F. is a co-founder, director, equity holder, and scientific advisor of MelioLabs, Inc. The interests of MelioLabs are not related to the contents of this paper.
Figures





Update of
-
Divergent iron-regulatory states contribute to heterogeneity in breast cancer aggressiveness.bioRxiv [Preprint]. 2024 Feb 13:2023.06.23.546216. doi: 10.1101/2023.06.23.546216. bioRxiv. 2024. Update in: iScience. 2024 Aug 03;27(9):110661. doi: 10.1016/j.isci.2024.110661. PMID: 37425829 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

