High-performance hydrogen evolution reaction in quadratic nodal line semimetal Na2CdSn
- PMID: 39262793
- PMCID: PMC11387805
- DOI: 10.1016/j.isci.2024.110708
High-performance hydrogen evolution reaction in quadratic nodal line semimetal Na2CdSn
Abstract
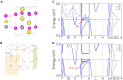
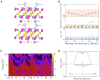
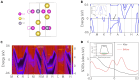
Topological nodal line semimetals (TNLSMs), which exhibit one-dimensional (1D) band crossing in their electronic band structure, have been predicted to be potential catalysts in electrocatalytic processes. However, the current studies are limited to the TNLSMs where the dispersion around the nodal line is linear in all directions. Here, the potential application of the quadratic nodal line (QNL) semimetal Na2CdSn in hydrogen evolution reaction is explored. Based on the bulk-boundary correspondence, we find that the topological surface states (TSSs) of the QNL are extended in the entire Brillouin zone. A linear relationship between the density of states of the TSSs and the Gibbs free energy is established in Na2CdSn. Remarkably, the best performance of Na2CdSn can be comparable to that of the noble metal Pt. Therefore, our work not only identifies an innovative type of topological catalyst with a QNL state but also confirms the relationship between TSSs and catalytic performance.
Keywords: Physics; Quantum physics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Zhang X., Yu Z.M., Zhu Z., Wu W., Wang S.S., Sheng X.L., Yang S.A. Nodal loop and nodal surface states in Ti3Al family materials. Phys. Rev. B. 2018;97 doi: 10.1103/PhysRevB.97.235150. - DOI
-
- Wu W., Liu Y., Li S., Zhong C., Yu Z.M., Sheng X.L., Zhao Y.X., Yang S.A. Nodal surface semimetals: Theory and material realization. Phys. Rev. B. 2018;97 doi: 10.1103/PhysRevB.97.115125. - DOI
-
- Meng W., Zhang X., Liu Y., Dai X., Liu G. Antiferromagnetism caused by excess electrons and multiple topological electronic states in the electride Ba4Al5·e−. Phys. Rev. B. 2021;104 doi: 10.1103/PhysRevB.104.195145. - DOI
LinkOut - more resources
Full Text Sources

