Effect of intrapartum azithromycin on gut microbiota development in early childhood: A post hoc analysis of a double-blind randomized trial
- PMID: 39262807
- PMCID: PMC11387895
- DOI: 10.1016/j.isci.2024.110626
Effect of intrapartum azithromycin on gut microbiota development in early childhood: A post hoc analysis of a double-blind randomized trial
Abstract
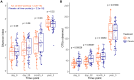
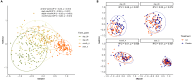
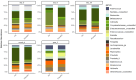
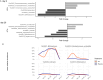
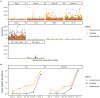
Intrapartum azithromycin prophylaxis has shown the potential to reduce maternal infections but showed no effect on neonatal sepsis and mortality. Antibiotic exposure early in life may affect gut microbiota development, leading to undesired consequences. Therefore, we here assessed the impact of 2 g oral intrapartum azithromycin on gut microbiota development from birth to the age of 3 years, by 16S-rRNA gene profiling of rectal samples from 127 healthy Gambian infants selected from a double-blind randomized placebo-controlled clinical trial (PregnAnZI-2). Microbiota trajectories showed, over the first month of life, a slower community transition and increase of Enterobacteriaceae (p = 0.001) and Enterococcaceae (p = 0.064) and a decrease of Bifidobacterium (p < 0.001) in the azithromycin compared to the placebo arm. Intrapartum azithromycin alters gut microbiota development and increases proinflammatory bacteria in the first month of life, which may have undesirable effects on the child.
Keywords: Health sciences; Medical microbiology; Medical specialty; Medicine; Microbiome; Pharmacology.
© 2024 Published by Elsevier Inc.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures

References
-
- Africa’s Advances in Maternal, Infant Mortality Face Setbacks: WHO Report. WHO; 2022. https://www.afro.who.int/news/africas-advances-maternal-infant-mortality... Regional Office for Africa.
-
- World Health Organization . Who; 2022. Newborn Mortality; pp. 56–59.
-
- Sharrow D., Hug L., You D., Alkema L., Black R., Cousens S., Croft T., Gaigbe-Togbe V., Gerland P., Guillot M., et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health. 2022;10:e195–e206. doi: 10.1016/S2214-109X(21)00515-5. - DOI - PMC - PubMed
-
- Hug L., Alexander M., You D., Alkema L., UN Inter-agency Group for Child Mortality Estimation National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob. Health. 2019;7:e710–e720. doi: 10.1016/S2214-109X(19)30163-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

