In situ deposition of nanobodies by an engineered commensal microbe promotes survival in a mouse model of enterohemorrhagic E. coli
- PMID: 39262854
- PMCID: PMC11388102
- DOI: 10.1093/pnasnexus/pgae374
In situ deposition of nanobodies by an engineered commensal microbe promotes survival in a mouse model of enterohemorrhagic E. coli
Abstract
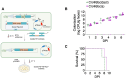
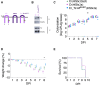
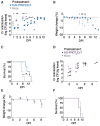
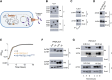
Engineered smart microbes that deliver therapeutic payloads are emerging as treatment modalities, particularly for diseases with links to the gastrointestinal tract. Enterohemorrhagic Escherichia coli (EHEC) is a causative agent of potentially lethal hemolytic uremic syndrome. Given concerns that antibiotic treatment increases EHEC production of Shiga toxin (Stx), which is responsible for systemic disease, novel remedies are needed. EHEC encodes a type III secretion system (T3SS) that injects Tir into enterocytes. Tir inserts into the host cell membrane, exposing an extracellular domain that subsequently binds intimin, one of its outer membrane proteins, triggering the formation of attaching and effacing (A/E) lesions that promote EHEC mucosal colonization. Citrobacter rodentium (Cr), a natural A/E mouse pathogen, similarly requires Tir and intimin for its pathogenesis. Mice infected with Cr(ΦStx2dact), a variant lysogenized with an EHEC-derived phage that produces Stx2dact, develop intestinal A/E lesions and toxin-dependent disease. Stx2a is more closely associated with human disease. By developing an efficient approach to seamlessly modify the C. rodentium genome, we generated Cr_Tir-MEHEC(ΦStx2a), a variant that expresses Stx2a and the EHEC extracellular Tir domain. We found that mouse precolonization with HS-PROT3EcT-TD4, a human commensal E. coli strain (E. coli HS) engineered to efficiently secrete an anti-EHEC Tir nanobody, delayed bacterial colonization and improved survival after challenge with Cr_Tir-MEHEC(ΦStx2a). This study suggests that commensal E. coli engineered to deliver payloads that block essential virulence determinants can be developed as a new means to prevent and potentially treat infections including those due to antibiotic resistant microbes.
Keywords: EHEC; T3SS; smart microbe; therapeutic E coli.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures
Update of
-
In situ deposition of nanobodies by an engineered commensal microbe promotes survival in a mouse model of enterohemorrhagic E. coli.bioRxiv [Preprint]. 2024 Jul 30:2024.07.30.605899. doi: 10.1101/2024.07.30.605899. bioRxiv. 2024. Update in: PNAS Nexus. 2024 Sep 02;3(9):pgae374. doi: 10.1093/pnasnexus/pgae374. PMID: 39131305 Free PMC article. Updated. Preprint.
Similar articles
-
In situ deposition of nanobodies by an engineered commensal microbe promotes survival in a mouse model of enterohemorrhagic E. coli.bioRxiv [Preprint]. 2024 Jul 30:2024.07.30.605899. doi: 10.1101/2024.07.30.605899. bioRxiv. 2024. Update in: PNAS Nexus. 2024 Sep 02;3(9):pgae374. doi: 10.1093/pnasnexus/pgae374. PMID: 39131305 Free PMC article. Updated. Preprint.
-
Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice.Mol Microbiol. 2003 Apr;48(1):95-115. doi: 10.1046/j.1365-2958.2003.03429.x. Mol Microbiol. 2003. PMID: 12657048
-
Synchronous Disease Kinetics in a Murine Model for Enterohemorrhagic E. coli Infection Using Food-Borne Inoculation.Front Cell Infect Microbiol. 2016 Nov 3;6:138. doi: 10.3389/fcimb.2016.00138. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27857935 Free PMC article.
-
Citrobacter rodentium(ϕStx2dact), a murine infection model for enterohemorrhagic Escherichia coli.Curr Opin Microbiol. 2022 Feb;65:183-190. doi: 10.1016/j.mib.2021.11.013. Epub 2021 Dec 17. Curr Opin Microbiol. 2022. PMID: 34929548 Free PMC article. Review.
-
Interkingdom Chemical Signaling in Enterohemorrhagic Escherichia coli O157:H7.Adv Exp Med Biol. 2016;874:201-13. doi: 10.1007/978-3-319-20215-0_9. Adv Exp Med Biol. 2016. PMID: 26589220 Review.
Cited by
-
Living Engineered Bacterial Therapeutics: Emerging Affordable Precision Interventions.Microb Biotechnol. 2024 Nov;17(11):e70057. doi: 10.1111/1751-7915.70057. Microb Biotechnol. 2024. PMID: 39579048 Free PMC article. Review.
References
-
- Cubillos-Ruiz A, et al. 2021. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov. 20:941–960. - PubMed
-
- Lodinová-Zádniková R, Sonnenborn U. 1997. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol Neonate. 71:224–232. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

