Off-the-shelf medication transformed: Custom-dosed metoprolol tartrate tablets via semisolid extrusion additive manufacturing and the perception of this technique in a hospital context
- PMID: 39263003
- PMCID: PMC11388020
- DOI: 10.1016/j.ijpx.2024.100277
Off-the-shelf medication transformed: Custom-dosed metoprolol tartrate tablets via semisolid extrusion additive manufacturing and the perception of this technique in a hospital context
Abstract
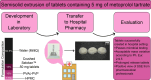
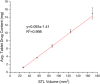
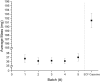
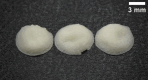
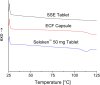
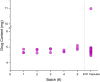
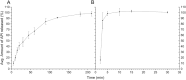
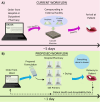
Pharmacies are currently unable to stock proper oral dosage forms for pediatric populations. This leads to manipulation of medications or the need to compound specialized medications, which can be a time-consuming process. Using Semisolid Extrusion (SSE) additive manufacturing (AM), specialized medications can be produced in an expedited process from off-the shelf medication in a hospital or outpatient pharmacy setting. In this study, tablets with a desired dose of 5 mg of metoprolol tartrate derived from commercial Seloken™ 50 mg tablets were 3D printed in a hospital setting. Validation testing was done on five batches, highlighting tablets with a high uniformity in mass and dimension, drug content, acceptable microbial assays, and prolonged release during in-vitro analysis. The average drug content found for the tablets was within ±6% of 5 mg for all batches produced. Comparisons were done between the SSE tablets and capsules produced in an external compounding facility, highlighting several positive aspects of SSE-produced tablets beyond simply shortening the production timeline. The SSE tablets printed in this study are characterized by their smaller size, enhanced prolonged release properties, and more uniform drug content across the tested samples. Additionally, interviews with pharmaceutical professionals were conducted to determine the positive aspects of SSE and further improvements to bring this technique as seamlessly as possible into the pharmacy. This study underscores the feasibility of employing SSE in the production of specialized medications within a hospital environment. Furthermore, it highlights the methodological advantages SSE offers over existing production standards, demonstrating its potential to improve pharmaceutical manufacturing in healthcare settings.
Keywords: 3D printing; Additive manufacturing; Dose modification; Semisolid Extrusion.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Jonas Lindh reports financial support was provided by Sweden's Innovation Agency. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Braun V., Clarke V. SAGE; 2022. Thematic Analysis: A Practical Guide.
-
- Bühler V. BASF SE; 1992. Kollidon: Polyvinylpyrrolidone for the Pharmaceutical Industry.https://books.google.se/books?id=1lYOtwAACAAJ
LinkOut - more resources
Full Text Sources

