Construction and drug screening of Co-culture system using extrahepatic cholangiocarcinoma organoids and tumor-associated macrophages
- PMID: 39263166
- PMCID: PMC11388766
- DOI: 10.1016/j.heliyon.2024.e36377
Construction and drug screening of Co-culture system using extrahepatic cholangiocarcinoma organoids and tumor-associated macrophages
Abstract
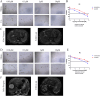
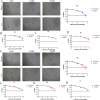
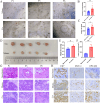
Patient-derived organoids (PDOs) have been proposed as a novel in vitro tumor model that can be applied to tumor research and drug screening. However, current tumor organoid models lack components of the tumor microenvironment, particularly tumor-associated macrophages(TAMs).We collected peripheral blood and tumor samples from 6 patients with extrahepatic cholangiocarcinoma(eCCA). Monocytes were induced into TAMs by cytokine and conditioned medium, and then co-cultured with tumor organoids. Our comprehensive analysis and comparison of histopathology and genomics results confirmed that this co-culture model can better capture intra- and inter-tumor heterogeneity retain the specific mutations of the original tumor. Drug sensitivity data in vitro revealed that gemcitabine and cisplatin are effective drugs for cholangiocarcinoma, but TAMs in the tumor microenvironment promote organoids growth and chemotherapy resistance. In conclusion, our organoid model of cholangiocarcinoma co-cultured with TAMs can not only shorten the model construction cycle, but also preserve the heterogeneity of original tumors to improve the accuracy of drug screening, and can also be applied to the researches of TAMs and tumors.
Keywords: Extrahepatic cholangiocarcinoma; Genetic profiles; Organoids; Therapy resistance; Tumor-associated macrophages.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Bertuccio P., Malvezzi M., Carioli G., Hashim D., Boffetta P., El-Serag H.B., La Vecchia C., Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019;71(1):104–114. - PubMed
-
- Tacke F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017;66(6):1300–1312. - PubMed
LinkOut - more resources
Full Text Sources

