Changes in short-chain fatty acids affect brain development in mice with early life antibiotic-induced dysbacteriosis
- PMID: 39263295
- PMCID: PMC11384438
- DOI: 10.21037/tp-24-128
Changes in short-chain fatty acids affect brain development in mice with early life antibiotic-induced dysbacteriosis
Abstract
Background: Early enteral nutrition and the gut microbiota profoundly influence neonatal brain development, with short-chain fatty acids (SCFAs) from the microbiota playing a pivotal role. Understanding the relationship between dysbiosis, SCFAs, and brain development is crucial. In this study, we investigated the impact of antibiotics on the concentration of SCFAs in neonatal feces. Additionally, we developed a model of gut dysbiosis in neonatal mice to examine the potential relationship between this imbalance, SCFAs production, and brain function development.
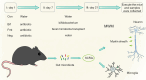
Methods: We measured the SCFAs content in the feces of two groups of neonates, categorized based on whether antibiotics were used, and conducted the Neonatal Behavioral Neurological Assessment (NBNA) test on all neonates. Then we evaluated fecal SCFAs levels in neonates and neonatal mice post-antibiotic treatment using liquid chromatography-mass spectrometry (LC-MS) analysis. Morris water maze (MWM) tests assessed behavioral performance, and western blot analysis examined brain tissue-related proteins-neuron-specific enolase (NSE), ionized calcium binding adaptor molecule-1 (IBA1), and myelin basic proteins (MBP).
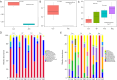
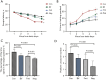
Results: The use of antibiotics did not affect the NBNA scores of the two groups of neonates, but it did reduce the SCFAs content in their feces. Antibiotic administration induced gut dysbiosis in mice, resulting in decreased IBA1 and MBP expression. Interventions to restore gut microbiota ameliorated these effects. Mice with dysbiosis displayed cognitive deficits in the MWM test. SCFAs levels decreased during dysbiosis, and increased upon microbiota recovery.
Conclusions: Neonatal dysbiosis affects the microbiota-gut-brain axis, impairing cognitive function and nervous system development. Reduced SCFAs may contribute significantly to these alterations.
Keywords: Gastrointestinal microbiome; ionized calcium binding adaptor molecule-1 (IBA1); myelin basic proteins (MBP); neuron-specific enolase (NSE); short-chain fatty acid (SCFA).
2024 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-128/coif). The authors have no conflicts of interest to declare.
Figures
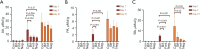


References
LinkOut - more resources
Full Text Sources
Miscellaneous
