Effect of ginseng and ginsenosides on attention deficit hyperactivity disorder: A systematic review
- PMID: 39263306
- PMCID: PMC11385392
- DOI: 10.1016/j.jgr.2024.05.006
Effect of ginseng and ginsenosides on attention deficit hyperactivity disorder: A systematic review
Abstract
Attention deficit hyperactivity disorder (ADHD) is a rapidly increasing neurodevelopmental disorder but currently available treatments are associated with abuse risk, side effects, and incomplete symptom relief. There is growing interest in exploring complementary options, and ginseng has gained attention for its therapeutic potential. This systematic review aimed to assess current evidence on the efficacy of ginseng and its active components, ginsenosides, for ADHD. Eligible studies were identified through searches of PubMed, Embase, Cochrane Library, and Web of Science, up to June 2023. The inclusion criteria included both human and animal studies that investigated the effects of ginseng or ginsenosides on ADHD. The risk of bias was assessed according to study type. Six human studies and three animal studies met the inclusion criteria. The results suggest that ginseng and ginsenosides may have beneficial effects on ADHD symptoms, particularly inattention, through dopaminergic/norepinephrinergicmodulation and BDNF/TrkB signaling. Ginseng and ginsenosides have promising potential for ADHD treatment. Due to limitations in evidence quality, such as the risk of bias and variability in study designs, larger controlled studies are essential. Integrating ginseng into ADHD management may have valuable implications for individuals seeking well-tolerated alternatives or adjunctive therapies.
Keywords: Attention-deficit hyperactivity disorder; Dopamine; Ginseng; Ginsenoside; Systematic review.
© 2024 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
There are no conflicts of interest to declare.
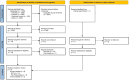
Figures



References
-
- Arnold L.E., Hodgkins P., Kahle J., Madhoo M., Kewley G. Long-term outcomes of ADHD: academic achievement and performance. J Atten Disord. 2020;24(1):73–85. - PubMed
-
- Faraone S.V., Banaschewski T., Coghill D., Zheng Y., Biederman J., Bellgrove M.A., Newcorn J.H., Gignac M., Al Saud N.M., Manor I., et al. The world federation of ADHD international Consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789–818. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

