Adhesion of retinal cells to gold surfaces by biomimetic molecules
- PMID: 39263323
- PMCID: PMC11387177
- DOI: 10.3389/fcell.2024.1438716
Adhesion of retinal cells to gold surfaces by biomimetic molecules
Abstract
Background: Neural cell-electrode coupling is crucial for effective neural and retinal prostheses. Enhancing this coupling can be achieved through surface modification and geometrical design to increase neuron-electrode proximity. In the current research, we focused on designing and studying various biomolecules as a method to elicit neural cell-electrode adhesion via cell-specific integrin mechanisms.
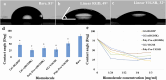
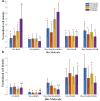
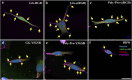
Methods: We designed extracellular matrix biomimetic molecules with different head sequences (RGD or YIGSR), structures (linear or cyclic), and spacer lengths (short or long). These molecules, anchored by a thiol (SH) group, were deposited onto gold surfaces at various concentrations. We assessed the modifications using contact angle measurements, fluorescence imaging, and X-ray Photoelectron Spectroscopy (XPS). We then analyzed the adhesion of retinal cells and HEK293 cells to the modified surfaces by measuring cell density, surface area, and focal adhesion spots, and examined changes in adhesion-related gene and integrin expression.
Results: Results showed that YIGSR biomolecules significantly enhanced retinal cell adhesion, regardless of spacer length. For HEK293 cells, RGD biomolecules were more effective, especially with cyclic RGD and long spacers. Both cell types showed increased expression of specific adhesion integrins and proteins like vinculin and PTK2; these results were in agreement with the adhesion studies, confirming the cell-specific interactions with modified surfaces.
Conclusion: This study highlights the importance of tailored biomolecules for improving neural cell adhesion to electrodes. By customizing biomolecules to foster specific and effective interactions with adhesion integrins, our study provides valuable insights for enhancing the integration and functionality of retinal prostheses and other neural implants.
Keywords: RGD; YIGSR; biomimetics; cell-adhesion; neural electrode interface; regenerative medicine; retinal prostheses; tissue engineering.
Copyright © 2024 Shpun, Markus, Farah, Zalevsky and Mandel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Ben-Shalom R., Keeshen C. M., Berrios K. N., An J. Y., Sanders S. J., Bender K. J. (2017). Opposing effects on NaV1.2 function underlie differences between SCN2A variants observed in individuals with autism spectrum disorder or infantile seizures. Biol. Psychiatry 82, 224–232. 10.1016/j.biopsych.2017.01.009 - DOI - PMC - PubMed
-
- Blau A. (2013). Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: an introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 18, 481–492. 10.1016/j.cocis.2013.07.005 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

