Duodenal-Jejunal Bypass Restores Sweet Taste Receptor-Mediated Glucose Sensing and Absorption in Diabetic Rats
- PMID: 39263491
- PMCID: PMC11390237
- DOI: 10.1155/2024/5544296
Duodenal-Jejunal Bypass Restores Sweet Taste Receptor-Mediated Glucose Sensing and Absorption in Diabetic Rats
Abstract
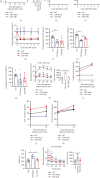
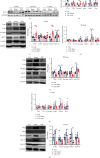
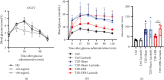
Aim: The aim of the study is to identify the regulatory role of intestinal sweet taste receptors (STRs) and glucose transporters (SGLT1, GLUT2) and gut peptide secretion in duodenal-jejunal bypass (DJB)-ameliorated glycemic control in Type 2 diabetes. Materials and Methods: DJB and sham surgeries were performed in streptozotocin-induced diabetic male rats. The blood GLP-1 and GLP-2 levels were evaluated under feeding and fasting conditions. The expression of STRs (T1R2, T1R3), sweet taste signaling effector (Gα-gustducin), SGLT1, and GLUT2 was detected in the intestinal alimentary limb (A limb), biliopancreatic limb (BP limb), and common limb (C limb). The effects of STR inhibition on glucose control were measured with lactisole. Results: Glucose tolerance was improved in DJB-operated rats compared with the sham group, similar to that of normal control rats, without significant differences in food intake and body weight. The plasma GLP-1 levels of DJB rats were increased under diet-fed condition, and GLP-2 levels were increased after fasting. The villus height and crypt depth were significantly increased in the A limb of DJB-operated rats. In addition, GLP-1 expression was restored in enterocytes. The expression of T1R2, Gα-gustducin, and SGLT1 was elevated in the A limb after DJB, while GLUT2 was downregulated in the A, BP, and C limbs. The localization of GLUT2 was normalized in the three intestinal limbs after DJB. However, the beneficial effects of DJB on glucose control were abolished in the presence of lactisole in vivo. Conclusion: DJB ameliorates glycemic control probably by restoring STR-mediated glucose sensing and absorption with the responses of GLP-1 and GLP-2 to carbohydrate.
Keywords: DJB; GLP-1; GLUT2; T1R2; lactisole.
Copyright © 2024 Sipeng Sun et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Role of Proximal Intestinal Glucose Sensing and Metabolism in the Blood Glucose Control in Type 2 Diabetic Rats After Duodenal Jejunal Bypass Surgery.Obes Surg. 2022 Apr;32(4):1119-1129. doi: 10.1007/s11695-021-05871-3. Epub 2022 Jan 26. Obes Surg. 2022. PMID: 35080701
-
Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes.Ann Surg. 2013 Jul;258(1):89-97. doi: 10.1097/SLA.0b013e3182890311. Ann Surg. 2013. PMID: 23478528
-
Expression of sweet taste receptor and gut hormone secretion in modelled type 2 diabetes.Gen Comp Endocrinol. 2017 Oct 1;252:142-149. doi: 10.1016/j.ygcen.2017.08.008. Epub 2017 Aug 4. Gen Comp Endocrinol. 2017. PMID: 28782537
-
Expedited Biliopancreatic Juice Flow to the Distal Gut Benefits the Diabetes Control After Duodenal-Jejunal Bypass.Obes Surg. 2015 Oct;25(10):1802-9. doi: 10.1007/s11695-015-1633-7. Obes Surg. 2015. PMID: 25726319
-
Glucose sensing and signalling; regulation of intestinal glucose transport.Proc Nutr Soc. 2011 May;70(2):185-93. doi: 10.1017/S0029665111000103. Epub 2011 Mar 30. Proc Nutr Soc. 2011. PMID: 21450125 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

