Krüppel-like factors family in health and disease
- PMID: 39263604
- PMCID: PMC11387732
- DOI: 10.1002/mco2.723
Krüppel-like factors family in health and disease
Abstract
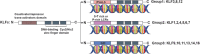
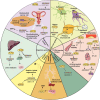
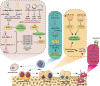
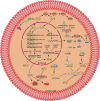
Krüppel-like factors (KLFs) are a family of basic transcription factors with three conserved Cys2/His2 zinc finger domains located in their C-terminal regions. It is acknowledged that KLFs exert complicated effects on cell proliferation, differentiation, survival, and responses to stimuli. Dysregulation of KLFs is associated with a range of diseases including cardiovascular disorders, metabolic diseases, autoimmune conditions, cancer, and neurodegenerative diseases. Their multidimensional roles in modulating critical pathways underscore the significance in both physiological and pathological contexts. Recent research also emphasizes their crucial involvement and complex interplay in the skeletal system. Despite the substantial progress in understanding KLFs and their roles in various cellular processes, several research gaps remain. Here, we elucidated the multifaceted capabilities of KLFs on body health and diseases via various compliable signaling pathways. The associations between KLFs and cellular energy metabolism and epigenetic modification during bone reconstruction have also been summarized. This review helps us better understand the coupling effects and their pivotal functions in multiple systems and detailed mechanisms of bone remodeling and develop potential therapeutic strategies for the clinical treatment of pathological diseases by targeting the KLF family.
Keywords: Krüppel‐like factors (KLFs); bone destruction diseases; bone homeostasis; energy metabolism; epigenetic modification; systemic diseases.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Xie W, Li L, Zheng XL, Yin WD, Tang CK. The role of Krüppel‐like factor 14 in the pathogenesis of atherosclerosis. Atherosclerosis. 2017;263:352‐360. - PubMed
-
- Lin Z, Kumar A, SenBanerjee S, et al. Kruppel‐like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96(5):e48‐e57. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
