Polymeric bis(triphenylphosphine)iminium chloride as a recyclable catalyst
- PMID: 39263665
- PMCID: PMC11382542
- DOI: 10.1039/d4sc03119a
Polymeric bis(triphenylphosphine)iminium chloride as a recyclable catalyst
Abstract
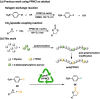
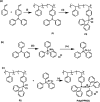
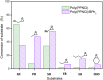
Metal-free catalysts have garnered considerable interest as an environmental and economical alternative to precious metal catalysts. Bis(triphenylphosphine)iminium chloride (PPNCl) has emerged as a prominent choice due to its air and thermal stability and broad reactivity, especially in applications where a bulky cation is needed. The high phosphorus content and synthetic effort required for catalyst synthesis increase environmental impact; the recyclability of PPNCl in catalytic processes remains largely unexplored. The potential development of a polymer-supported PPNCl catalysts therefore desirable to enable this recyclability. In this work, we synthesise polymeric PPNCl (poly(PPNCl)) for the first time. Poly(PPNCl) demonstrates a comparative catalytic reactivity to its small molecule variant when employed as a catalyst in halogen-exchange reactions and CO2/epoxide coupling. For the latter the effect of catalyst loading, CO2 pressure, reaction time and addition of co-catalyst on conversion and selectivity was investigated. Poly(PPNCl) was easily recovered from the crude product by simple precipitation and its catalytic reactivity was well-maintained over three reaction cycles, providing environmental and economic advantages for sustainable reaction development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

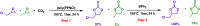

References
-
- Saini N. Malik A. Jain S. L. Light driven chemical fixation and conversion of CO2 into cyclic carbonates using transition metals: A review on recent advancements. Coord. Chem. Rev. 2024;502:215636. doi: 10.1016/j.ccr.2023.215636. - DOI
-
- Wang X. Li Z. Qu Y. Yuan T. Wang W. Wu Y. Li Y. Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chem. 2019;5:1486–1511.
-
- Esteves H. Morais Costa N. E. Tomazett V. K. Milani J. L. S. das Chagas R. P. de Fátima Â. Organocatalysis for the Chemical Fixation of Carbon Dioxide to Synthesise N-Heterocycles. Curr. Green Chem. 2024;11:87–126. doi: 10.2174/0122133461255437230930182648. - DOI
-
- Yang D. Gates B. C. Catalysis by metal organic frameworks: perspective and suggestions for future research. ACS Catal. 2019;9:1779–1798. doi: 10.1021/acscatal.8b04515. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

