Point-based risk score for the risk stratification and prediction of hepatocellular carcinoma: a population-based random survival forest modeling study
- PMID: 39263676
- PMCID: PMC11388332
- DOI: 10.1016/j.eclinm.2024.102796
Point-based risk score for the risk stratification and prediction of hepatocellular carcinoma: a population-based random survival forest modeling study
Abstract
Background: The precise associations between common clinical biomarkers and hepatocellular carcinoma (HCC) risk remain unclear but hold valuable insights for HCC risk stratification and prediction.
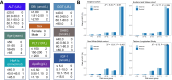
Methods: We examined the linear and nonlinear associations between the baseline levels of 32 circulating biomarkers and HCC risk in the England cohort of UK Biobank (UKBB) (n = 397,702). The participants were enrolled between 2006 and 2010 and followed up to 31st October 2022. The primary outcome is incident HCC cases. We then employed random survival forests (RSF) to select the top ten most informative biomarkers, considering their association with HCC, and developed a point-based risk score to predict HCC. The performance of the risk score was evaluated in three validation sets including UKBB Scotland and Wales cohort (n = 52,721), UKBB non-White-British cohort (n = 29,315), and the Taizhou Longitudinal Study in China (n = 17,269).
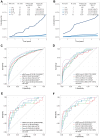
Findings: Twenty-five biomarkers were significantly associated with HCC risk, either linearly or nonlinearly. Based on the RSF model selected biomarkers, our point-based risk score showed a concordance index of 0.866 in the England cohort and varied between 0.814 and 0.849 in the three validation sets. HCC incidence rates ranged from 0.95 to 30.82 per 100,000 from the lowest to the highest quintiles of the risk score in the England cohort. Individuals in the highest risk quintile had a 32-73 times greater risk of HCC compared to those in the lowest quintile. Moreover, over 70% of HCC cases were detected in individuals within the top risk score quintile across all cohorts.
Interpretation: Our simple risk score enables the identification of high-risk individuals of HCC in the general population. However, including some biomarkers, such as insulin-like growth factor 1, not routinely measured in clinical practice may increase the model's complexity, highlighting the need for more accessible biomarkers that can maintain or improve the predictive accuracy of the risk score.
Funding: This work was supported by the National Natural Science Foundation of China (grant numbers: 82204125) and the Science and Technology Support Program of Taizhou (TS202224).
Keywords: Cohort study; Common clinical biomarkers; HCC; Nonlinear correlation; Point-based scoring system.
© 2024 The Author(s).
Conflict of interest statement
All authors declare no conflict of interests.
Figures



References
-
- Fedirko V., Duarte-Salles T., Bamia C., et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60:1222–1230. - PubMed
-
- Åberg F., Helenius-Hietala J., Puukka P., et al. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–2149. - PubMed
LinkOut - more resources
Full Text Sources

