Renal Mast Cell-Specific Proteases in the Pathogenesis of Tubulointerstitial Fibrosis
- PMID: 39263893
- PMCID: PMC11529666
- DOI: 10.1369/00221554241274878
Renal Mast Cell-Specific Proteases in the Pathogenesis of Tubulointerstitial Fibrosis
Abstract
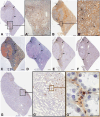
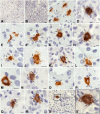
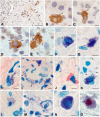
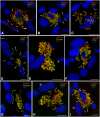
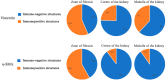
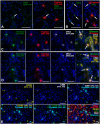
Chronic kidney disease is detected in 8-15% of the world's population. Along with fibrotic changes, it can lead to a complete loss of organ function. Therefore, a better understanding of the onset of the pathological process is required. To address this issue, we examined the interaction between mast cells (MCs) and cells in fibrous and intact regions, focusing on the role of MC proteases such as tryptase, chymase, and carboxypeptidase A3 (CPA3). MCs appear to be involved in the development of inflammatory and fibrotic changes through the targeted secretion of tryptase, chymase, and CPA3 to the vascular endothelium, nephron epithelium, interstitial cells, and components of intercellular substances. Protease-based phenotyping of renal MCs showed that tryptase-positive MCs were the most common phenotype at all anatomic sites. The infiltration of MC in different anatomic sites of the kidney with an associated release of protease content was accompanied by a loss of contact between the epithelium and the basement membrane, indicating the active participation of MCs in the formation and development of fibrogenic niches in the kidney. These findings may contribute to the development of novel strategies for the treatment of tubulointerstitial fibrosis.
Keywords: carboxypeptidase A3; chymase; fibrogenic niche; kidney fibrosis; mast cells; specific tissue microenvironment; tryptase.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

