Enhancing Cardioprotection Through Neutrophil-Mediated Delivery of 18β-Glycyrrhetinic Acid in Myocardial Ischemia/Reperfusion Injury
- PMID: 39264272
- PMCID: PMC11558124
- DOI: 10.1002/advs.202406124
Enhancing Cardioprotection Through Neutrophil-Mediated Delivery of 18β-Glycyrrhetinic Acid in Myocardial Ischemia/Reperfusion Injury
Abstract
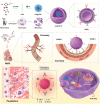
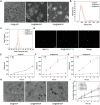
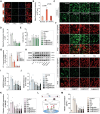
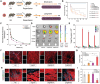
Myocardial ischemia/reperfusion injury (MI/RI) generates reactive oxygen species (ROS) and initiates inflammatory responses. Traditional therapies targeting specific cytokines or ROS often prove inadequate. An innovative drug delivery system (DDS) is developed using neutrophil decoys (NDs) that encapsulate 18β-glycyrrhetinic acid (GA) within a hydrolyzable oxalate polymer (HOP) and neutrophil membrane vesicles (NMVs). These NDs are responsive to hydrogen peroxide (H2O2), enabling controlled GA release. Additionally, NDs adsorb inflammatory factors, thereby reducing inflammation. They exhibit enhanced adhesion to inflamed endothelial cells (ECs) and improved penetration. Once internalized by cardiomyocytes through clathrin-mediated endocytosis, NDs protect against ROS-induced damage and inhibit HMGB1 translocation. In vivo studies show that NDs preferentially accumulate in injured myocardium, reducing infarct size, mitigating adverse remodeling, and enhancing cardiac function, all while maintaining favorable biosafety profiles. This neutrophil-based system offers a promising targeted therapy for MI/RI by addressing both inflammation and ROS, holding potential for future clinical applications.
Keywords: drug delivery; inflammation; myocardial ischemia‐reperfusion injury; nanomedicine; neutrophil decoys (NDs); reactive oxygen species (ROS).
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gong F. F., Vaitenas I., Malaisrie S. C., Maganti K., JAMA Cardiol. 2021, 6, 341. - PubMed
-
- Bhatt D. L., Lopes R. D., Harrington R. A., JAMA 2022, 327, 662. - PubMed
-
- Reed G. W., Rossi J. E., Cannon C. P., Lancet 2017, 389, 197. - PubMed
-
- Sabatine M. S., Braunwald E., J. Am. Coll. Cardiol. 2021, 77, 2822. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
