Determination of Initial Rates of Lipopolysaccharide Transport
- PMID: 39264328
- PMCID: PMC11447908
- DOI: 10.1021/acs.biochem.4c00379
Determination of Initial Rates of Lipopolysaccharide Transport
Abstract
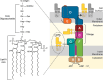
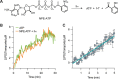
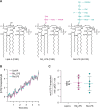
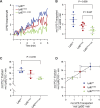
Nonvesicular lipid trafficking pathways are an important process in every domain of life. The mechanisms of these processes are poorly understood in part due to the difficulty in kinetic characterization. One important class of glycolipids, lipopolysaccharides (LPS), are the primary lipidic component of the outer membrane of Gram-negative bacteria. LPS are synthesized in the inner membrane and then trafficked to the cell surface by the lipopolysaccharide transport proteins,
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Combining Mutations That Inhibit Two Distinct Steps of the ATP Hydrolysis Cycle Restores Wild-Type Function in the Lipopolysaccharide Transporter and Shows that ATP Binding Triggers Transport.mBio. 2019 Aug 20;10(4):e01931-19. doi: 10.1128/mBio.01931-19. mBio. 2019. PMID: 31431556 Free PMC article.
-
Suppressor Mutations in LptF Bypass Essentiality of LptC by Forming a Six-Protein Transenvelope Bridge That Efficiently Transports Lipopolysaccharide.mBio. 2023 Feb 28;14(1):e0220222. doi: 10.1128/mbio.02202-22. Epub 2022 Dec 21. mBio. 2023. PMID: 36541759 Free PMC article.
-
Outer Membrane Translocon Communicates with Inner Membrane ATPase To Stop Lipopolysaccharide Transport.J Am Chem Soc. 2018 Oct 10;140(40):12691-12694. doi: 10.1021/jacs.8b07656. Epub 2018 Sep 28. J Am Chem Soc. 2018. PMID: 30253645 Free PMC article.
-
Structural insight into lipopolysaccharide transport from the Gram-negative bacterial inner membrane to the outer membrane.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Nov;1862(11):1461-1467. doi: 10.1016/j.bbalip.2017.08.003. Epub 2017 Aug 15. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28821406 Review.
-
Structural Insights into the Lipopolysaccharide Transport (Lpt) System as a Novel Antibiotic Target.J Microbiol. 2024 Apr;62(4):261-275. doi: 10.1007/s12275-024-00137-w. Epub 2024 May 31. J Microbiol. 2024. PMID: 38816673 Review.
References
-
- Rothfield L. I.Biological membranes: An overview at the molecular level. In Structure and Function of Biological Membranes; Academic Press, 1971; pp 3–9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

