Loss of cardiomyocyte-specific adhesion G-protein-coupled receptor G1 (ADGRG1/GPR56) promotes pressure overload-induced heart failure
- PMID: 39264336
- PMCID: PMC11427730
- DOI: 10.1042/BSR20240826
Loss of cardiomyocyte-specific adhesion G-protein-coupled receptor G1 (ADGRG1/GPR56) promotes pressure overload-induced heart failure
Abstract
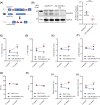
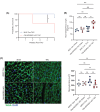
Adhesion G-protein-coupled receptors (AGPCRs), containing large N-terminal ligand-binding domains for environmental mechano-sensing, have been increasingly recognized to play important roles in numerous physiologic and pathologic processes. However, their impact on the heart, which undergoes dynamic mechanical alterations in healthy and failing states, remains understudied. ADGRG1 (formerly known as GPR56) is widely expressed, including in skeletal muscle where it was previously shown to mediate mechanical overload-induced muscle hypertrophy; thus, we hypothesized that it could impact the development of cardiac dysfunction and remodeling in response to pressure overload. In this study, we generated a cardiomyocyte (CM)-specific ADGRG1 knockout mouse model, which, although not initially displaying features of cardiac dysfunction, does develop increased systolic and diastolic LV volumes and internal diameters over time. Notably, when challenged with chronic pressure overload, CM-specific ADGRG1 deletion accelerates cardiac dysfunction, concurrent with blunted CM hypertrophy, enhanced cardiac inflammation and increased mortality, suggesting that ADGRG1 plays an important role in the early adaptation to chronic cardiac stress. Altogether, the present study provides an important proof-of-concept that targeting CM-expressed AGPCRs may offer a new avenue for regulating the development of heart failure.
Keywords: ADGRG1; Adhesion GPCR; GPR56; cardiomyocytes; heart failure; pressure overload.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

